High boiled sweets
To produce high boiled sweets with a satisfactory shelf life the final product must contain a minimum amount of residual moisture and the correct balance between sucrose and the glucose syrup/invert sugar components. To achieve this, sucrose and glucose syrup/invert sugar solutions are heated and boiled down to a set solids or moisture content, appropriate flavorings, colors and fruit acids being added during the boiling process. The boiling point is obviously affected by the solids level and the higher the boiling point, the higher the residual solids in the product. Under vacuum, the boiling point of an equivalent solids level or concentration is reduced and this principle is used in the food industry to some advantage. It is not possible to produce high-boiled confections of adequate shelf life from sucrose solution alone and glucose syrup/invert sugar must be added for the reasons discussed below.
The main problem during the storage of high boiled sweets is the formation of small crystals, commonly known as graining which renders the product unacceptable. This is due to the recrystallization of sucrose, which in its simplest form may be considered as the migration of molecules to a nucleus.
High boiled sweets are a classic example of a product in the ‘glassy’ state. In appearance they are solid, but actually they are supercooled, noncrystalline liquids, which are so far below their melting or softening point that they have assumed solid properties without crystallizing. They can be considered as liquids with very high viscosities, a property that interferes considerably with the process of crystal formation. For crystallization to occur there must be a ‘nucleus’, that is, a completely sub-microscopic crystal to act as a ‘seed’. These nuclei are formed spontaneously if the supersaturation is sufficiently high, but the higher the viscosity the slower the rate at which they form. Molecules of the substance crystallizing have to locate and stick to the nucleus, being brought to it by their continuous, rapid vibrational movement. This movement is severely limited in a solid or in a liquid of extremely high viscosity.
To obtain a product of satisfactory texture and shelf life a solids level of 97% must be achieved, however, such a system based on sucrose alone would be supersaturated with respect to this carbohydrate making it unstable. This problem can be overcome by using, for example, a 60:40 blend of sucrose and glucose syrup. The glucose syrup increases the viscosity of the confection in its ‘glassy’ state, and thus reduces the mobility of the lower molecular weight carbohydrates. The viscosity of glucose syrup (at constant solids content) depends on its composition and particularly on the concentration of high molecular weight components. At the solids content of high boiled sweets, the effect of the higher saccharides is significant. Furthermore, the viscosity of the boiling mass in the pan and during its subsequent handling can be significantly affected by quite small variations in the amount of high molecular weight components in the syrup. In addition this variation can also affect the ‘break’ of the sweet in the mouth, that is, its brittleness and toughness.
The stability with regard to moisture pick-up or loss by any food product depends upon its ERH or relative vapor pressure. The ERH of high boiled sweets is around 30%, and since atmospheric humidity is nearly always above this there is always a tendency for the sweets to absorb moisture. Initially, this only takes place on the surface; a very thin film of a solution with a solids content significantly lower than that of the remainder of the sweet is formed, which also has a much lower viscosity. In this film the reduced solids level can permit the crystallization of sucrose (graining) to take place, which can then spread into the body of the sweet. There is some evidence to show that the presence of glucose syrup can impart a ‘skin’ to the outer surface of the sweet, which greatly inhibits the penetration of moisture to the interior. Glucose syrup, when used for the above reasons, is referred to as a ‘doctor’. Invert sugar can also be used for the same purpose but is not so efficient.
Certain high boiled sweets depend upon graining actually being induced· to produce a particular texture, e.g. rock. The mechanical action of pulling is used to beat in air and precipitate very fine sucrose crystals. Fondant is also a grained confection in which the amount of ‘doctor’ is much lower and the viscosity is higher because of the increased solids content.
The production of the recently available, all-enzyme processed glucose syrups presented the food manufacturers, in this case of high boiled sweets, with some interesting raw materials, in particular, high maltose syrups which are characterized by their low glucose and high maltose contents and low viscosities compared with acid and acid-enzyme syrups of the same DE. The effect of the different carbohydrate spectra of 42 DE glucose syrups on their viscosities is shown in Figure 1.
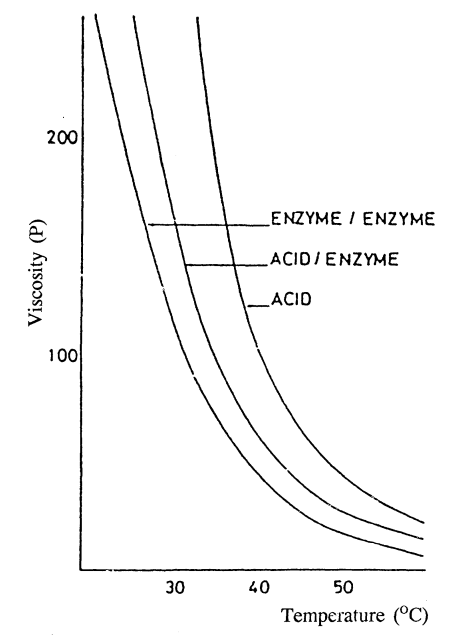
A useful application of these differing viscosities is that the viscosities of high boiling sugar (candy) masses of given sucrose composition can be reduced in the manufacturing process giving a better deposition performance and better (lower) air retention. Other advantages are that the mass is easier to work on a slab, does not form a hard outside crust so easily, and the final sweets are more plastic during forming with better surface flow, resulting in a higher gloss and improved finished appearance.
Conversely, similar handling characteristics can be obtained at high glucose syrup concentrations. Another useful effect is that the boiling point elevation is influenced by the glucose syrup concentration and type. This shows the relationship between boiling temperature, at constant pressure, and residual solids for different ratios of sucrose and glucose syrups. It can be seen that by using the correct glucose syrup and syrup ratio, the boiling temperature can be reduced by as much as 5°C. This becomes of increasing importance to food manufacturers as the cost of energy rises.
High maltose syrups show less brown color formation on heating as a direct result of their low glucose content. This is important in boiled sweets where discolorations are undesirable. The color developed on heating glucose syrups depends on a number of factors. The first is the concentration of trace color bodies or color precursors, for example, hydroxy methyl furfural (HMF) and amino acids which may be present in the syrup, and the second is the reducing sugar content. The majority of colors are produced as a result of the Maillard reaction and simple sugars, such as glucose, undergo the reaction most readily.
Production of high boiled sweets
There are three main production methods used for high boiled sweets, namely open pan, vacuum cookers and continuous cookers. Each of these requires a different optimum ratio of sucrose to glucose syrup to give the best results and to maintain product quality by preventing sucrose crystallization. The reason for an increased glucose syrup content in boiling is twofold. First, as methods become more advanced the amount of agitation to which the syrup is subjected is greatly increased, and second, the amount of time for which the syrup is subjected to high temperatures is considerably reduced, thus lessening the danger of inversion. It should be noted that these comments only apply to the more traditional glucose syrups, such as the acid and acid-enzyme converted products rather than the new enzyme-enzyme syrups.
Open pan boiling is limited in application since at the high temperatures involved, discoloration of the syrup may occur. Vacuum cooking overcomes this problem since the temperature at which the same total solids content is obtained is lower. Open pan methods can produce syrups containing 95-96% solids whereas with vacuum methods a total solids of around 98-99% can be achieved. The higher the solids content the longer the shelf life of the product. With vacuum cooking excessive agitation of the syrup at the predetermined temperature for sweet production should be avoided since this may induce sucrose crystallization. Syrup feed rate, steam pressure, and precook temperature should remain constant to maintain the quality of the product.
Continuous methods of manufacture often use a microfilm-type cooker which is basically a scraped film evaporator. Heat is supplied by superheated steam and sucrose/glucose syrup solution is introduced continuously at the top of the cooker. The sugar is cooked in a turbulent film about 1 mm thick in about 8 s and is not discoloured by heating.
After cooking the sugar masses from the above processes are discharged and further processed into their final forms ready for distribution and sale.
Toffees, caramel and fudge
This family of confectionery products is characterized by the development of color and flavor by the encouragement of the Maillard browning reaction with the milk proteins. As a rule, therefore, acid-converted syrups containing higher levels of dextrose are preferred but it is possible to alter processing conditions to accommodate high maltose syrups in some cases, particularly where their lower relative candy viscosity is desirable in high-speed processes. Also, a lighter color can be desirable in some instances to give a creamy appearance. The difference between caramels and toffee lies normally in the moisture content of the products and their fat content. Both are essentially complexes offat globules surrounded by a concentrated sugar solution containing non-milk fat solids. The sucrose/lactose/ glucose syrup ratio is of prime importance with regard to the keeping properties of the product. Acid-converted 42 DE glucose syrup is usually used although the viscosity, color development, and firmness of the product may be changed by using a different glucose syrup. The higher the concentration of the glucose syrup the greater the stretch properties of the caramel and toffee. Higher DE syrups increase flowability and maltose syrups produce caramels that are light in color and creamy in appearance.
The presence of milk solids causes the product to be different in its properties from other types of confection. During processing, the milk protein and the reducing sugars may undergo the Maillard reaction, the resulting compounds contributing to the overall color and flavor of the caramel or toffee. The reaction is fastest at temperatures in excess of 95°C, and the higher the temperature and the longer the time of heating the, greater the flavor development and darker the color. The caramelization of the sugars also occurs at high temperatures and the end products of this reaction also contribute to the caramel flavor and color. A typical caramel formulation is shown in Table 1. The fat is usually a mixture of hardened palm kernel oil and butterfat. Small amounts of salt and flavoring are also usually added.
| Proportion required (no. of parts) | Ingredient |
| 4 | Brown sugar |
| 10 | White granulated sugar |
| 14 | Glucose syrup |
| 10 | Sweetened condensed milk |
| 10 | Hard toffee fat (in small pieces) |
| 2 | Dairy butter |
| 0.015 | Glyceryl monostearate or lecithin |
Gums and jellies
This is a popular class of confection in the USA and Europe and is gaining popularity in other parts of the world. Basically, these are low boiled confections with a residual moisture of about 20% and whose major textural characteristics are given by the hydrocolloid used. Hydrocolloids are commonly starch, gelatine, agar, pectin, and gum arabic. With the exception of gum arabic in gum confections where it is used at 35-45%, the hydrocolloid comprises less than 10% and the balance is largely glucose syrup and sucrose. Although the major properties are exerted by the hydrocolloid, the selection and level of glucose syrup still has a part to play in the properties of this class of product since it is included at 30-50% of the formulation.
Traditionally 42 DE acid-converted glucose syrup is used in these products to give protection against the crystallization of the sucrose. If, however, tender eating properties are required the gel properties can be improved by the use of enzyme high maltose syrups where the reduction in higher sugar causes less stickiness and a cleaner gel performance. The higher sugars cause problems with pectin and gelatine gels as they interfere with the macromolecular set of the hydrocolloid. Where starch is the hydrocolloid, this of course does not apply and in starch-based pastilles, for example, 36 DE glucose syrup is used to assist the viscosity of the system. In pectin jellies where 42 DE glucose can cause toughness in the gel, again high maltose syrup or even 63 DE glucose syrup may be used to overcome this and also to give increased sweetness in the case of the 63 DE glucose syrup.
Marshmallow
Marshmallows is an aerated sugar-containing foam, stabilized with gelatine and egg albumen. Gum arabic and starch may be added. It is produced by a cold process in which a sugar syrup (previously prepared by boiling) and a solution of a whipping agent are mixed and aerated. A 63 DE glucose syrup is normally used because it has good humectant and whipping properties. Invert sugar, glucose or sorbitol may also be added. High maltose syrups are also used owing to their low viscosity and high moisture retention properties. They will also help prevent glucose crystallization.
Nougat
Nougat is basically a high boiled syrup containing fat to which is added a frappe, containing either egg albumen or gelatine. Whipping agents, starch, gum arabic or agar may also be added as texture modifiers. For chewy, non-grained, non-chocolate enrobed nougat, a 36-38 DE or high maltose syrup is used to maintain viscosity in the syrup phase and help prevent stickiness. For non-enrobed, short textured and grained nougat a traditional 42 DE glucose syrup is used to increase moisture retention. For chocolate covered nougat a 63 DE syrup is used to provide considerably more moisture retention and reduce drying out on storage.
Chewing gum
Chewing gum is another confectionery item widely produced both in Europe and the United States. The most common form is the stick pack. Increasingly sugar-free chewing gum is being produced using sugar alcohols. Traditional chewing gum consisted of a sugar impregnated into gum base. Glucose syrup typically provided some 25% of this sugar. The type of glucose syrup used was acid-converted 42 DE or 35 DE, the higher sugars adding to the chewiness of the texture and the slow and sustained release of the sugar. The most significant factor which characterizes glucose syrup for chewing gum is the concentration, usually 44-45°Bé and 85% dry solids. This is because the chewing gum process is not a boiling process and any water added has to be minimized.
