Thermal Stability of Chemically Modified Starch
The thermal stability of chemically modified starch was characterized by thermogravimetric analysis (TGA) in a nitrogen atmosphere. Figure 4.5 shows how the thermal degradation temperature of a starch was changed by chemical modification. The thermal degradation temperature is defined by the temperature at which the weight loss of a sample reaches 10 wt % in the weight loss versus temperature curves obtained from TGA measurements.
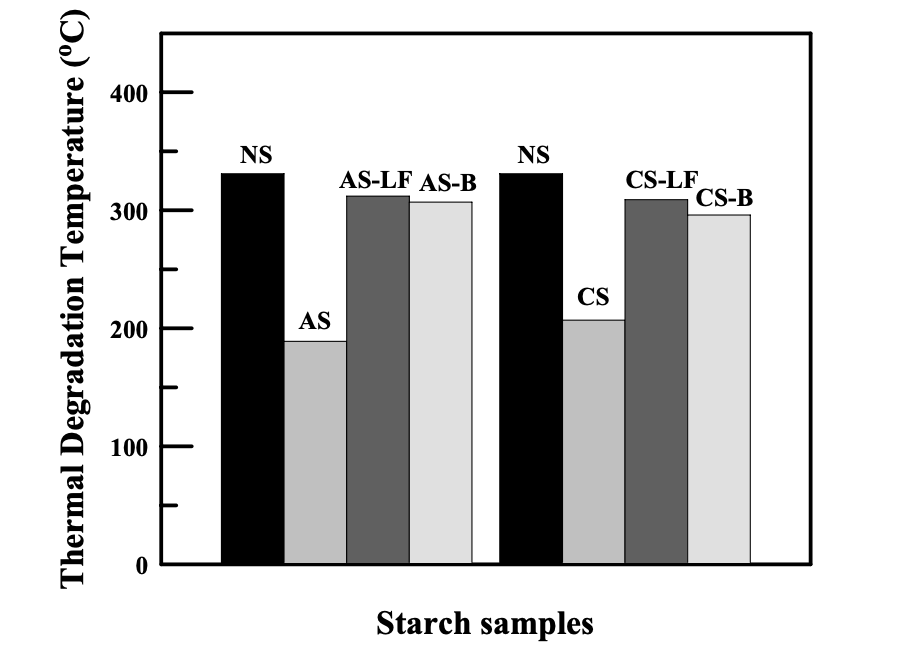
It is worth noting that the thermal degradation temperature of starch greatly decreased from 330 o C (natural starch) to around 200 o C after modification with ionic groups (AS or CS). Previous studies73 on the thermal stability of starch showed that the
thermal weight loss of starch started with the intra- and intermolecular dehydration among hydroxyl groups and then was followed by decomposition and depolymerization at a higher temperature. In both AS and CS, only a very small amount of hydroxyl groups were substituted by ionic groups. The intra- and intermolecular dehydration among hydroxyl groups would not be hindered by the substitution of ionic groups. Moreover, the substitution of ionic groups destroyed the granular structure of natural starch and made abundant hydroxyl groups more easily accessible to each other for the dehydration.
On the other hand, Figure 4.5 shows that the thermal degradation temperatures of the starches modified with hexanoyl groups or benzoyl groups (AS-B, AS-LF, CS-B, or CS-LF) were almost not changed. In these modified starches, the DS of hexanoyl groups or benzoyl groups is around 2, suggesting that out of three hydroxyl groups on each repeat unit, two hydroxyl groups were substituted. The decreased total number of hydroxyl groups in the modified starch has lowered the possibility of having dehydration among the available hydroxyl groups. Furthermore, the hexanoyl groups or the benzoyl groups interfered with the accessibility of hydroxyl groups.
Glass Transition Temperature
As described in Thermoplastic Starch (TPS), the glass transition temperature of dried natural starch cannot be practically measured, because it is higher than the thermal decomposition temperature. It is necessary to lower the glass transition temperature of a starch below its thermal decomposition temperature so that we can possibly process starch as a conventional thermoplastic material. Thermoplastic starch can be prepared in two ways: one is by the addition of low-molecular-weight plasticizer, and the other is by chemical modification. In this dissertation, side groups such as hexanoyl groups and benzoyl groups were attached into a starch through chemical reaction with hydroxyl groups. Figures 4.6 and 4.7 give the DSC thermograms of the modified starches synthesized in this study. For starches modified only with ionic groups (AS or CS), there was no discernible glass transition temperature found in the DSC thermograms. For starches modified with multiple functional groups (AS-B, AS-LF, CS-B, or CS-LF), we can clearly observe the glass transition temperature in the DSC thermograms. The introduction of hexanoyl groups or benzoyl groups into a starch destroyed the semicrystalline granular structure and the hydrogen bonds formed between the hydroxyl groups. Hence, the mobility of starch was enhanced. In addition, the introduction of functional groups into a starch have increased the free volume of the modified starch. Thus, the glass transition temperature of a starch was lowered and it became discernible in the DSC measurements. On the other hand, for a starch modified only with ionic groups (AS or CS), the DS of ionic groups was around 0.1. Only a very small amount of hydroxyl groups was substituted by the ionic groups. There still are very strong hydrogen bonds in both AS and CS, which limit the mobility of the starch molecules. Thus, the glass transition temperature of AS or CS was not discernible in the DSC thermograms.
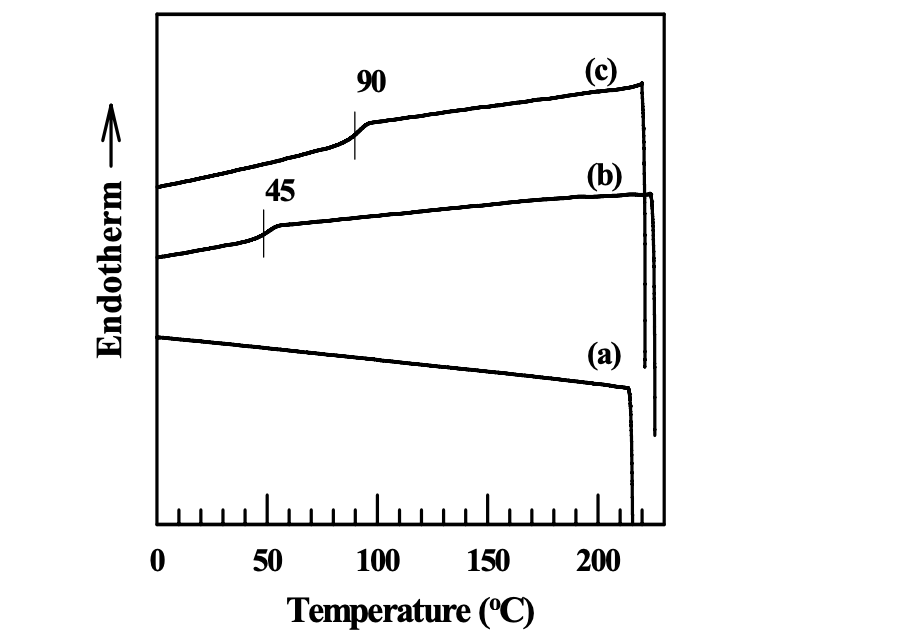
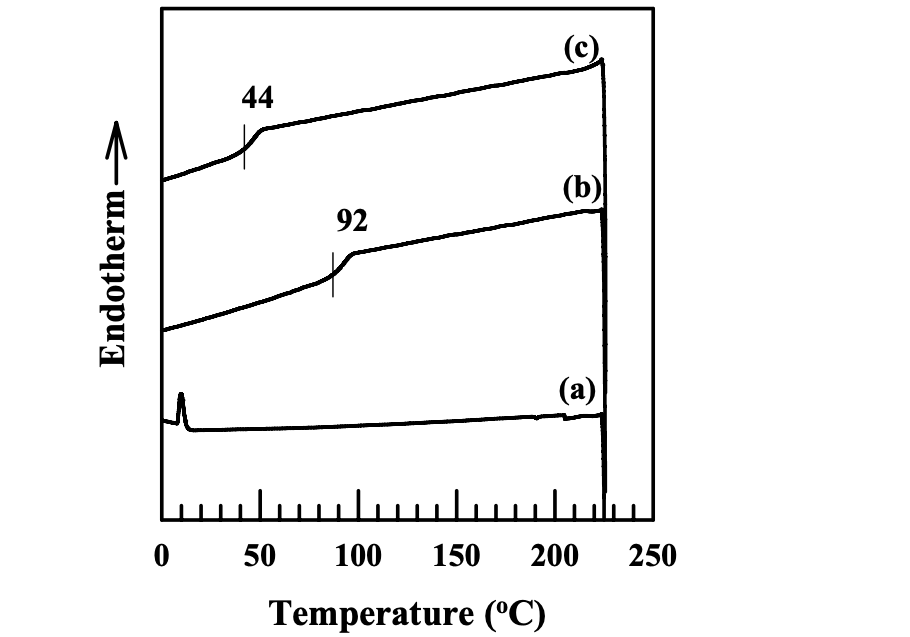
It should be noted that there was no melting peak on the DSC thermograms of modified starches, indicating an amorphous morphology in the modified starches. The modification by functional groups destroyed the semicrystalline granular structure of natural starch. Furthermore, the bulky side chains in the starch molecules after modification decreased the irregularity of starch molecules so that it was hard for modified starch to crystallize.
