As described in Introduction to natural starch, there are three hydroxyl groups in each repeat unit of starch molecules. Chemical modification explored in the present study is to substitute these hydroxyl groups with functional groups, such as anionic groups, cationic groups, hexanoyl groups, or benzoyl groups. The rationale behind this modification was as follows.
- Because of ionic interaction between the modified starch and clay, starch having anionic or cationic groups can have higher compatibility with organoclay or natural clay.
- Because the granular semicrystalline structure of natural starch was destroyed by the interruption of functional groups, starch having hexanoyl groups or benzoyl groups can have higher molecular mobility than natural starch.
- Because the hexanoyl groups or benzoyl groups are hydrophobic, starch having hexanoyl groups or benzoyl groups can have lower affinity with water than natural starch.
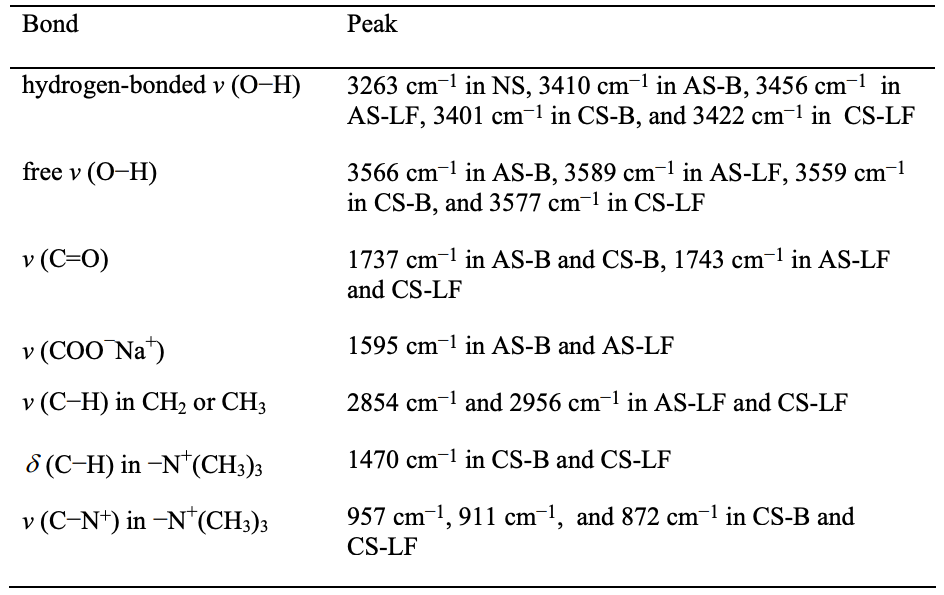
Figures 4.1 and 4.2 give the FTIR spectra of the modified starches synthesized in the present study. Following the FTIR spectra, Table 4.1 lists the major absorbance bands shown in the FTIR spectra for the functional groups in the modified starches.
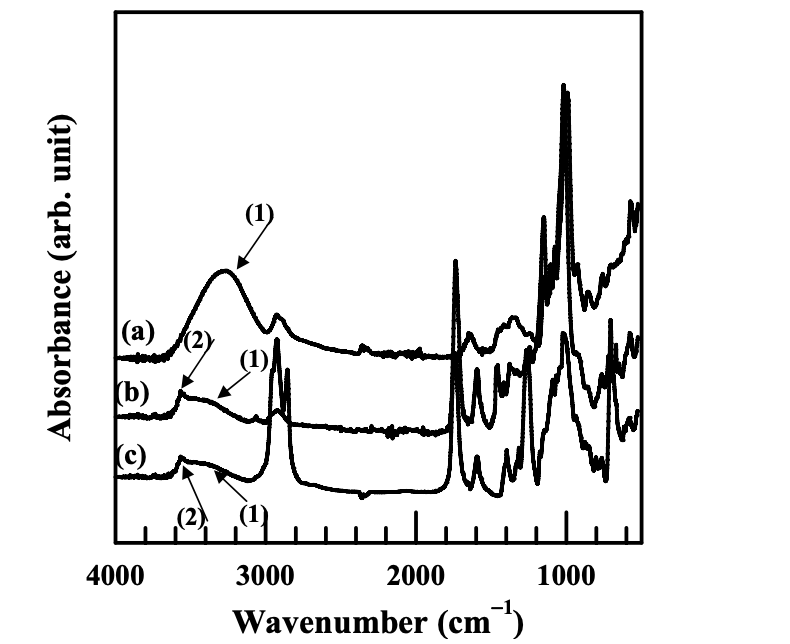
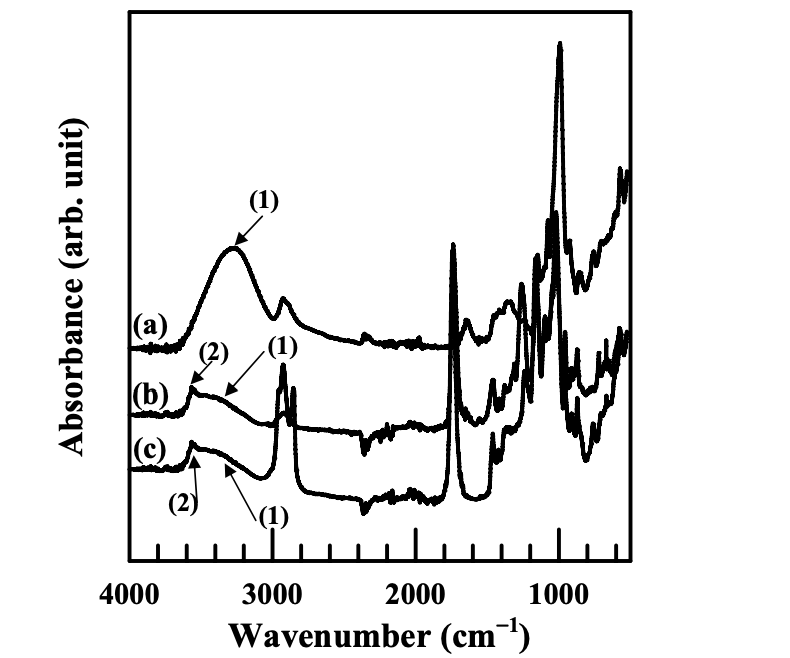
The FTIR spectra of natural starch (NS) show a very broad and strong band at 3263 cm−1, suggesting strong hydrogen bonds in natural starch. These hydrogen bonds are present in the granular structure of natural starch, in both the amorphous and crystalline phases. As described in Previous Research on the Chemical Modification of Starch, the mobility of starch molecular chains is very low (its glass transition temperature is higher than its thermal degradation temperature), which was partly resulting from the strong hydrogen bonds present among molecules. (The other reasons could include high molecular weight and microscopically granular structure of natural starch.)
The FTIR spectra of the modified starches synthesized in the present study include a starch modified with anionic group and benzoyl group (AS-B), a starch modified with anionic group and hexanoyl group (AS-LF), a starch modified with cationic group and benzoyl group (CS-B), and a starch modified with cationic group and hexanoyl group (CS-LF). They show that the absorbance band of hydrogen-bonded −OH group was lowered and shifted to a higher wavenumber, suggesting that the hydrogen bonds in a modified starch were drastically decreased as compared with natural starch. Furthermore, the absorbance band of free −OH group was observed in the FTIR spectra of a modified starch. The substitution of hydroxyl groups in a modified starch by other functional groups decreased the total number of hydroxyl group so that the strong hydrogen bonds were weakened. Additionally, the presence of functional groups, such as hexanoyl groups or benzoyl groups, interfere with the hydrogen bonds present between the available hydroxyl groups. That is the reason why we can observe the absorbance peaks of free −OH groups in a modified starch.
Qualitatively, we have shown above that chemical modification decreased the total number of hydroxyl groups in the starch molecules. The hydrogen bonds present in starch molecules were decreased, upon chemical modification, because of the lowered number of available hydroxyl groups. However, the number of hydroxyl groups substituted by functional groups and the number of hydroxyl groups available in a starch should be determined quantitatively. The values of degree of substitution (DS) indicate that the number of hydroxyl groups substituted in each repeat unit of a starch molecule ranges from 0 to 3. The DS of modified starches were determined from the 1 H-NMR spectra of a modified starch by equations 4.1 to 4.4:
DSanionic = Amethylene / 2Aanomeric (4.1)
DScationic = Amethyl / 9Aanomeric (4.2)
DShexanoyl = Amethyl / 3Aanomeric (4.3)
DSbenzoyl = Abenzoyl / 5Aanomeric (4.4)
in which A stands for the integrated area of chemical shift found from the 1 H-NMR spectra for specific functional groups. Figure 4.3 gives the 1 H-NMR spectra, showing how the DS of hexanoyl group was determined. The peak locating at 5.1 ppm is the chemical shift of anomeric proton in the glycosidic ring. The peaks locating around 1.1 ppm are the chemical shift of methyl protons in hexanoyl group. The numbers under both of those two peaks denote the integrated peak area (Aanomeric and Amethyl). The value of DS for all modified starches were determined in the same way. Table 4.2 lists the values of DS for the four chemically modified starches.
| Sample code | DSa1 | DSa2 |
| AS-B | 0.03 | 1.3 |
| AS-LF | 0.03 | 1.2 |
| CS-B | 0.1 | 1.7 |
| CS-LF | 0.03 | 1.6 |
a DS1 is the degree of substitution of ionic group in modified starch, and DS2 is the degree of substitution of hexanoyl groups or benzoyl groups in modified starch.
