The thermal characteristics of chemically modified starch can be studied by DSC, which measures the energy absorbed as a function of temperature. And by analyzing the endothermal curve, the onset temperature (To), peak temperature (Tp), conclusion temperature (Tc), thermal transition temperature (Tc-To), and enthalpy (△H) can be obtained. The DSC studies have shown that chemical modification alters thermal transition (Tc-To) temperatures and the overall enthalpy (△Hgel) associated with gelatinization (Tables 1 and 2).
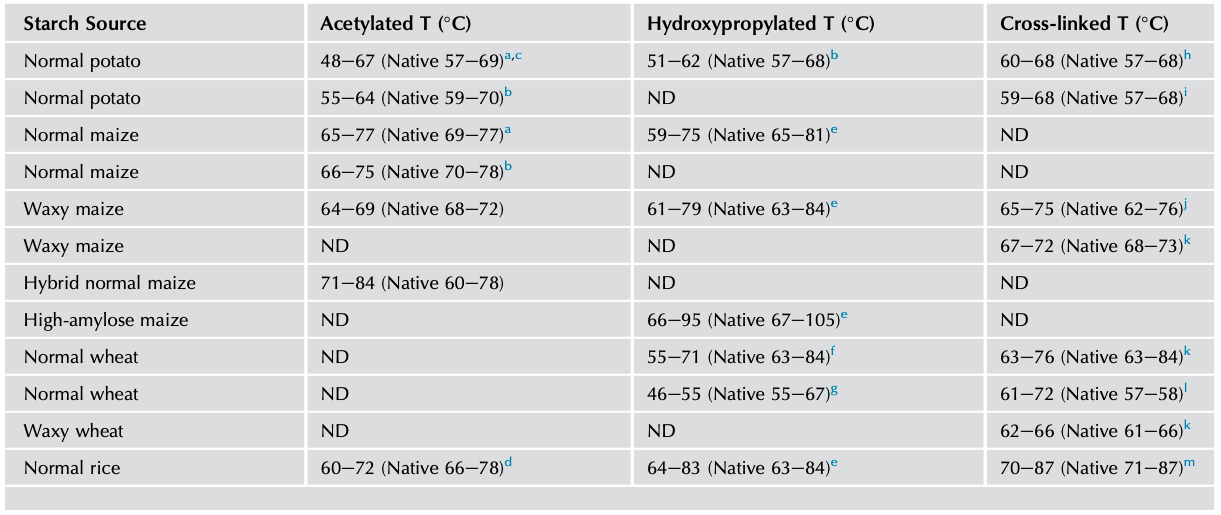
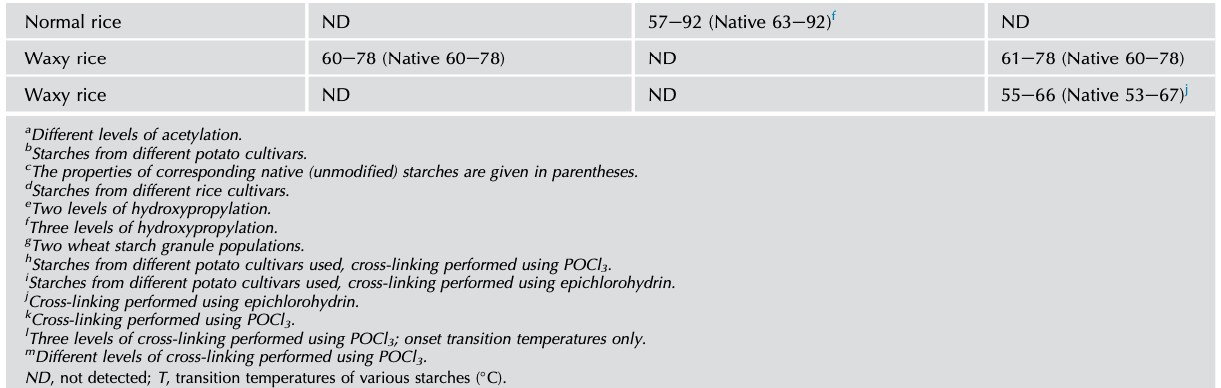
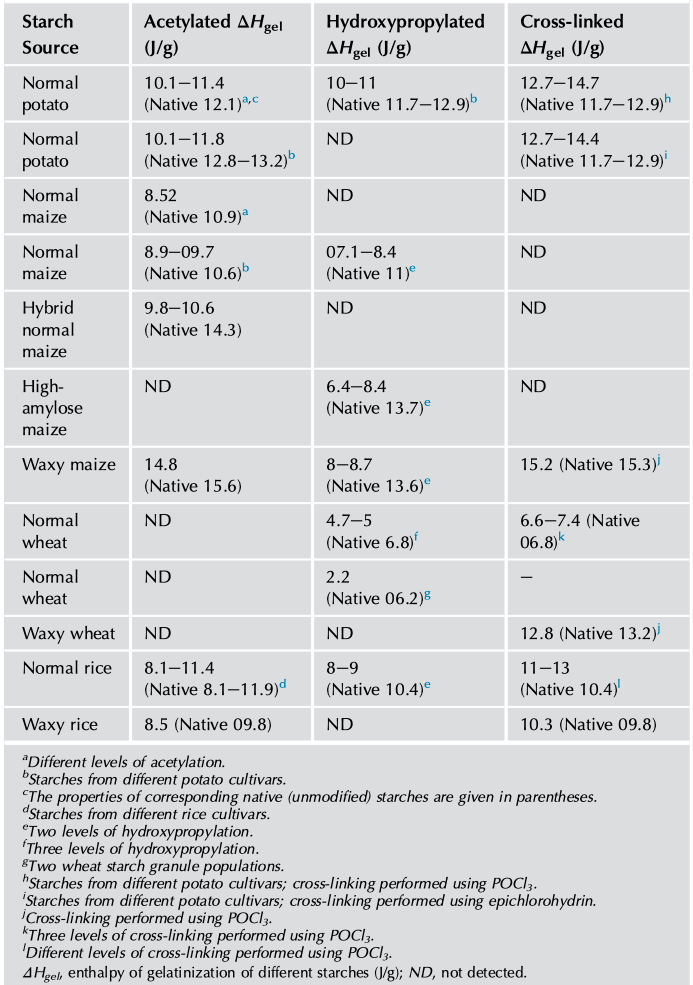
Acetylated potato, corn, and rice starches have been observed to show greater decreases in the transition temperatures and enthalpy of gelatinization (Colussi et al., 2014; Luo and Shi, 2012). Because the weakening of the starch granules by acetylation leads to early rupture of the amylopectin double helices, which accounts for the lower values of To, Tp, and Tc (Adebowale and Lawal, 2003). Owing to the damage to the crystals during acetylation, lowering of transition △Hgel has been observed and was different for varied botanical starches. Among these different starches, potato starch shows a greater decrease in thermal parameters upon acetylation compared with maize and rice starches. The differences in granule rigidity, presence/absence of lipids, DS, and amylose-to-amylopectin ratio between these starches affect the extent of changes in thermal characteristics (Kaur et al., 2004; Singh et al., 2004; Sodhi and Singh, 2005).
Hydroxypropylated starches showed a broadened transition temperature (Tc-To) and lowered enthalpy (△Hgel) with increasing MS indicating increased internal plasticization and destabilization of the amorphous regions of the granules. The functional groups introduced into the starch chains are capable of disrupting the inter- and intramolecular hydrogen bonds, leading to an increase in accessibility by water that lowers the temperature of gelatinization during hydroxypropylation. Consequently, the number of double helices that unravel and melt during gelatinization would be lower in hydroxypropylated than in unmodified starches (Perera et al., 1997; Seow and Thevamalar, 1993).
Cross-linking also alters the thermal transition characteristics of starch, the effect depending on the concentration and type of cross-linking reagent, reaction conditions, and botanical source of the starch (Singh et al., 2016). The level of phosphate cross-links has a strong influence on thermal characteristic of starches. Cross-linked starches with a relatively low concentration of POCl3 had gelatinization parameters similar to those of native starches, while with higher reagent concentrations, they showed considerably higher Tc and △Hgel values because cross-linking at lower levels reduces the proportion of the starch that can be gelatinized and results in a lower value of △Hgel (Choi and Kerr, 2004; Majzoobi et al., 2015; Yook et al., 1993).
