Various methods have been developed for the determination of Resistant Starch (RS) involving significant differences in sample preparation, enzymes used, and the establishment of experimental conditions that mimic the gastrointestinal environment. Most methods have focused on the determination of total RS, but specific methods have been developed to quantify RS1, RS2, and RS3.
- Introduction to Resistant Starch
- Structure and Thermal Properties of Starch Granules
- 5 types of Resistant Starch (RS)
- Factors that affect the digestibility of starch
- Resistant Starch (RS) Preparation
- The Health Effects of Resistant Starch (RS)
- The Use of Resistant Starch (RS) in Foods
A basic method for the determination of Resistant Starch (RS) was proposed by Englyst et al. in 1982. Briefly, the homogenized or ground sample is heated for gelatinization, and then hydrolysis is performed with an amylase mixture at 40 °C for 16 h. The residue obtained by centrifugation is further solubilized and treated with amyloglucosidase at 65 °C. Then the amount of glucose in the supernatant is determined using gas-liquid chromatography. This protocol includes homogenization and boiling steps, which eliminate the contribution of RS1 and RS2 to the final RS; therefore the result represents only the amount of RS3 in the sample.
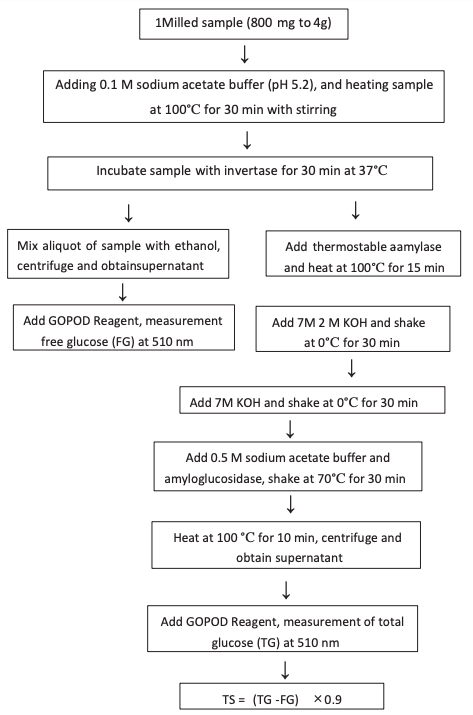
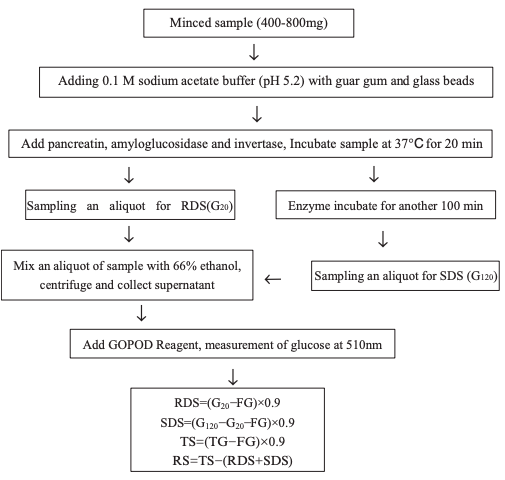
The above procedure was later modified by Englyst to measure total glucose (TG), free glucose (FG), total RS, and RS1, RS2, and RS3 as separate fractions, and the total starch (TS) was calculated as TS = (TG – FG)×0.9. In the revised protocol for TS determination, RS1 and RS2 were eliminated by mincing or milling the food sample and subsequently heating the sample at 100 °C for 30 min. Any retrograded starch (RS3) was eliminated by treating the sample with 7 M potassium hydroxide at 0 °C. The rapidly digestible starch (RDS) and SDS of the sample were determined separately, and the RS was calculated as RS = TS – (RDS+SDS). In this method, 0.8–4.0 g of sample was minced only to minimize structural changes. The low viscosity of the samples resulted in a higher release of glucose during enzyme hydrolysis than during in vivo digestion of starch. Guar gum was therefore added to increase the viscosity, and glass beads were added to ensure the breakage of cell walls during enzyme hydrolysis. The enzyme hydrolysis was performed at 37 °C, with sequential sampling aliquots of the enzyme digest at 20 and 120 min. The release of glucose reached a plateau at 120 min. The glucose content of the 20 min enzyme digest (G20 fraction) represented RDS and was calculated as RDS = (G20 – FG)×0.9. The released glucose between 20 and 20 min represented SDS and was calculated as SDS = (G120 – G20 – FG)×0.9. RS was calculated as RS = TS – (RDS +SDS). Using this protocol, 37 °C was the highest temperature at which the sample was treated. Any RS2 in the food sample was hydrolyzed by α-amylase, and RS1 was preserved in the enzyme digest because only minced pretreatment and no milling or homogenization were used. As a result, this indirect measurement of RS included any RS1, RS2, and RS3 in the sample (Figures 1 and 2).
When considering the possible effects of sample preparation such as drying, cooling, and the storage conditions on the level of Resistant Starch (RS) in RS protocols, Goni et al. further modified the method of Englyst et al by milling or homogenizing the samples before hydrolysis and decreasing the sample pH to 1.5 with HC1-KC1 buffer to simulate the gastric pH. Hydrolysis with pepsin at 40 °C for 1 h was then performed. To simulate conditions in the small intestine, the sample pH was adjusted to 6.9 with Tris-maleate buffer. Instead of using a mixture of enzymes, hydrolysis was performed with α-amylase for only 16 h at 37 °C.
The Megazyme assay kit (Irishtown, Ireland) is widely used in analytical laboratories for the determination of Resistant Starch (RS) and is the basis of both the AACC Method 32–40 and AOAC Method 2002.02. In the Megazyme protocol, the samples are ground to pass through a 1 mm sieve, and a mixture of α-amylase and amyloglucosidase is used at 37 °C for 16 h to hydrolyze starch in raw or processed food samples. Because there is no boiling treatment, RS2 in raw foods is not hydrolyzed during this procedure. After inactivation of enzymes with 99% ethanol, the pellet collected from the digestion is washed with 50% ethanol and treated with 2 M potassium hydroxide to extract RS3 from the fiber-rich matrix. The resulting dextrins are hydrolyzed to glucose with amyloglucosidase at 50 °C for 30 min. Finally, the oxidase-peroxidase colorimetric assay is used to determine the glucose concentration in the final hydrolysate. The Megazyme protocol for the determination of RS cannot be used for the determination of SDS and RDS.
Other modifications have been made in the procedure to determine the Resistant Starch (RS), especially in the sample preparation step with the intent of simulating in vivo digestion. However, significantly different levels of RS are detected in similar foods because of wide variations in analytical protocols, including differences in the enzymes used and in their activities, concentrations, sequences of applications, and dissimilarities in the conditions of the experimental protocols.
