Intestinal fermentation
In healthy humans, partial hydrolysis of disaccharide polyols occurs in the small intestine but absorption is incomplete and a mixture of hydrolysates and undigested polyol passes to the large bowel and is subject to bacterial fermentation (Billaux et al., 1991; Livesey, 1992). Monosaccharide alcohols are also incompletely absorbed and contribute to a carbohydrate pool which also includes unhydrolysed polyol, undigested starch, dietary fibre and endogenous mucins, and which is available for fermentation. The products of this fermentation are the gases H2 , CO2 , CH4 (in about onethird of the population) and organic acids, mainly the short chain fatty acids (SCFA) acetate, propionate and butyrate but also including succinate, formate and lactate as well as ethanol. Miller and Wolin (1979) calculated that in humans SCFA account for about 72% of the energy content of luminal carbohydrate excluding that used by the bacteria for growth. SCFA concentrations rise during the passage of intestinal contents from the terminal ileum (ca. 4 mM) into the colon (80 to 90 mM) from where they are absorbed by the epithelial cells at rapid rates ranging from 6 to 12 μmol cm-2 h-1 (McNeil et al., 1978). However, Hammer et al. (1989) demonstrated that the organic acids produced in diarrhoea induced by carbohydrate (lactulose) malabsorption were only partially absorbed in the colon and that unabsorbed organic acids, being anions, obligated the accumulation of inorganic cations (Na+ > Ca2+ > K+ > Mg2+) in the diarrhoeal fluid. In the case of low doses of lactulose, diarrhoea was induced by unabsorbed organic acids and associated cations whereas with larger doses of lactulose, unmetabolised carbohydrate also played a major role. Overall, however, in the case of lactulose the net effect of bacterial metabolism and the effect of partial absorption of organic acids on stool water output was dose dependent. The same is almost certainly true following the ingestion of other partially digestible carbohydrates such as the disaccharide alcohols. Those SCFAs which are absorbed then enter the general metabolism.
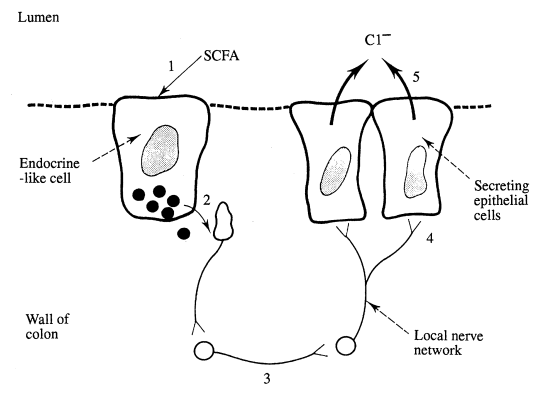
SCFAs have effects other than providing an energy source and influencing the movement of inorganic ions and water. Some can stimulate either colonic secretion, motility or epithelial cell division. For example an increase in Cl– secretion is produced by propionate, although not by acetate, perhaps by a mechanism similar to that by which secretagogues act in the small intestine (Johnson, 1991). It has been proposed that SCFAs activate endocrine-like cells which release peptides and/or amines into the interstitial fluid. As a result, nearby neurones are excited and a secretion elicited which releases acetyl choline close to the muscarinic cholinergic receptors on the basolateral membranes of secretory colonic cells. SCFAs also stimulate the growth of the colonic mucosa, butyrate being more potent than either acetate or propionate.
The ingestion of polyols, which are poorly absorbed, is thus associated with an increased concentration of carbohydrate entering the large bowel and with increased levels of colonic fermentation. If the fermentative capacity of the colon is exceeded osmotic diarrhoea occurs (Hammer et al., 1989; Rambaud and Flourie, 1994; Saunders and Wiggins, 1981) and is associated with a consequent increase in gastrointestinal symptomatology including colic, flatus and borborygms. Although polyols have a laxative effect when consumed in large amounts, this effect cannot be considered to be toxic and is modified by the colonic fermentation of the responsible sugar.
Stool consistency, laxation and diarrhoea
Grimble et al. (1988) found that 2/6 adapted subjects had diarrhoea after consumption of lactitol on an empty stomach. However, other studies have shown that if the dose is fractionated or is taken with meals laxativity is less pronounced (Patil et al., 1987; Van Es et al., 1986). Similarly, Beaugerie et al. (1989) did not observe laxation either in six healthy subjects after the intake of 20 g maltitol into an empty stomach or after the ingestion of three times higher doses when consumed by adapted subjects after eating three meals. The same can be said of isomalt (Beaugerie et at., 1989; Thiebaud et al., 1984) when consumed under similar conditions. However, there is no doubt that the ingestion of large enough doses of polyols can have a laxative effect. Gryboski (1966) found that sorbitol incorporated into candies caused osmotic diarrhoea when ingested at levels of 20 to 50 g. In a study in which volunteers were fed diets supplemented with either lactitol or saccharose, Van Es et al. (1986) claimed that sorbitol promotes osmotic diarrhoea whereas lactitol is fermented in the large intestine resulting in increased gas formation and the production of an increased faeces biomass. From a therapeutic standpoint, Heitland and Mauersberger (1988) found that 20 g of crystalline lactitol powder and 30 cm3 lactulose syrup were efficacious in increasing stool frequency. Similarly, Goovaerts and Ravelli (1993) reported the efficacy of lactitol for the treatment of chronic functional constipation, but found that with a dose of 20 g lactitol per day up to 46% of subjects experienced adverse gastrointestinal side effects such as abdominal cramps/pain, meteorism, flatulence and diarrhoea.
Thus, ingestion of sufficient amounts of non-absorbable or poorly absorbable, low molecular weight carbohydrates such as polyols may induce osmotic diarrhoea which originates in the small intestine as a result of profuse water and electroyte secretion into the duodenum and proximal jejunum (Rambaud and Flourie, 1994). The increased flow rate exceeds the adaptability of the intestine’s small diameter with a consequent shortening of oro-caecal transfer time which further decreases the hydrolysis and absorption of the already poorly absorbed carbohydrate (Kruger et at., 1992; Rambaud and Flourie, 1994). When the non-absorbed carbohydrates enter the colon they are fermented as outlined above (see ‘Intestinal fermentation’). The colonic metabolism of polyols into readily absorbable short chain fatty acids (SCFA) thus reduces the osmotic load which abrogates the potential diarrhoea which would otherwise have resulted from the high ileal output. McNeil (1984) pointed out that “all that enters the large intestine is not lost” and” … dietary carbohydrate, rather than leading to diarrhoea, is fermented in the colon and enhances the absorption of fluid and electrolytes”.
The overall laxative effect is thus determined by the slow, passive absorption rate of pentitols (xylitol) and hexitols (sorbitol and mannitol) and to the incomplete hydrolyis and absorption of polydextrose and disaccharide alcohols (isomalt, lactitol and maltitol). Sugars and sugar alcohols are osmotically active and thus influence the osmoregulatory capacity of the bowel. Although the colon is endowed with an extraordinary capacity to conserve fluid and electrolytes, its absorptive capacity can be exceeded in certain circumstances. Sugars such as glucose and fructose initially result in water transport into the gastrointestinal tract but they are rapidly absorbed by active and passive mechanisms, and so their osmotic effect is quickly dissipated. Xylitol, sorbitol and mannitol have the strongest osmotic effect of all the polyols followed by polydextrose, lactitol, isomalt and maltitol according to their molecular weight (Rambaud and Flourie, 1994)
Other gastrointestinal symptoms
Patil et al. (1987) have shown that higher doses of lactitol caused an increase in gastrointestinal side effects following the consumption of one or two boluses per day. In a double-blind, randomised crossover study on 21 normal subjects, Patil et al. (1987) determined the laxative threshold of lactitol as being 74 g per 25 h compared to 71 g for sorbitol; however the consumption of up to 40 g lactitol was possible without undesirable side effects. They concluded that the tolerance and laxative threshold of lactitol is similar to that of sorbitol and Patil et al. (1987) found that undesirable symptoms appeared at an intake of 40 g per day for both lactitol and sorbitol. Beaugerie et al. (1991) showed up to 84% malabsorption of an iso-osmotic solution containing 20 g lactitol without the appearance of intolerance symptoms.
Maltitol is reported to undergo a higher degree of small intestinal hydrolysis than lactitol and, as such, might be expected to cause a lower degree of osmotic diarrhoea and to stimulate fewer of the other symptoms of gastrointestinal intolerance than lactitol. Abraham et al. (1981) showed that the low calorie sweetener Marvie®, containing 58% maltitol, was tolerated without any gastrointestinal symptomatology when consumed at between 20 g and 30 g per day although above this level flatulence and abdominal discomfort occurred. Koizumi et at. (1983) estimated that a dose of 0.8 g kg-1 body weight was the 50% effective dose for laxation and abdominal symptomatology for adult Japanese subjects. Beaugerie et al. (1991) indicated 44% malabsorption of 20 g lactitol in iso-osmotic solution without the appearance of intolerance symptoms.
Isomalt contains equimolar quantities of mannitol and sorbitol and may be expected to provoke gastrointestinal intolerance symptoms according to its content of these two monosaccharide alcohols. Fritz et at. (1985) found that although there was no reported incidence of diarrhoea when 50 g isomalt was consumed, other intolerance symptoms such as abdominal noise, meteorism and flatulence did occur. The bacterial fermentation of isomalt and its hydrolysates may lead to gastrointestinal reactions if a threshold dose is exceeded and Spengler et al. (1987) noted that when healthy subjects were given increasing doses of isomalt in various foods there was a dose-dependent increase in flatulence over a 12 week period. It was concluded that daily doses of 48 g isomalt are tolerated well over an extended period of time. Similarly, daily doses of 24 g of isomalt taken over a 12 week period by Type II diabetics did not stimulate the appearance of intolerance symptoms (Porn etta and Trabichet, 1985). Furthermore, Beaugerie et at. (1991) showed up to 40% malabsorption of an iso-osmotic solution containing 20 g isomalt without any intolerance.
Biometric studies on polyols in confectionery items
A number of studies have been carried out at the University of Salford to investigate which of the new generation disaccharide alcohols and HGS are best tolerated when incorporated into confectionery items such as chewy sweets and milk chocolate and given to healthy young adult volunteers in properly controlled, randomized, double-blind, longitudinal or crossover field trials. Parameters which have been examined are single and multiple dose administration, acute and chronic consumption, consumption before and after overnight fasting and whether or not a dose-response effect exists. Symptoms which have been examined include flatulence, borborygmi and abdominal pain (colic) as well as stool consistency and frequency.
Hydrogenated glucose syrup in chewy sweets
One hundred healthy adults aged 18 to 35 years were invited to consume fruit-flavored chewy sweets containing either hydrogenated glucose syrup + mannitol (HGS/M) or glucose syrup + sucrose (GS/S) as bulk sweeteners. Products were presented as 50 g bags containing 10 X 5 g pieces, each containing a total of 0.4 g bulk sweetener, i.e. a total daily dose of 40 g. Subjects were asked to consume one packet of sweets per day for 14 days, spreading sweet intake ad libitum throughout the day. Milk and fruit juice consumption was restricted to 500 cm3 of each per day and beans were banned. The crossover design was double-blind and randomized with a wash-out period of a week between the two 14-day consumption periods. 91 subjects completed the study. Table 1 summarises the gastrointestinal side effects that occurred during the study period. Clearly, the HGS/M sweets provoked laxation and this was associated with an increased level of gastrointestinal symptomatology, in particular, increased flatus. However, the number of subjects passing loose stools on each day of the 14-day HGS/M sweet consumption period decreased and this decrease was highly significant when compared with the period during which GS/S were consumed. This trend is apparent whether the criterion of laxation is one or more, two or more or three or more loose motions per day. This decrease in the severity of laxation during the HGS-sweet consumption period indicates that acclimatization occurred during which the colonic flora presumably adapted to digest HGS more effectively. Consistent with these observations, Kearsley and Birch (1978) and Kearsley et al. (1982) showed that the repeated intake of 50 g Lycasin® during a single day on an empty stomach led to diarrhoea but Beaugerie et al. (1989) showed that adapted volunteers were symptomless following intake of 69 g per day of Lycasin® during meals. The osmotic effect of polyol or HGS thus depends on the dosage given and the degree of absorption which improves by an adaptive mechanism involving a hormonal effect.
| (a) HGS/M Sweets | (b) GS/S Sweets | |
| Motion frequency (number per day ± s.e.) | 1.41 ± 0.54 | 1.26 ± 0.48* |
| Number loose motions in study period (14 days) | 414 | 172** |
| Number of study days (of 14) when at least 1 loose motion passed | 304 | 137** |
| Number of subjects with at least 2 loose motions on 1 study day (of 14) | 33 | 9* |
| Flatus | more | less** |
| Colic | more | less** |
Follow-up studies at Salford were centred on a group of 100 subjects who consumed sweets containing either a moderate or a high level of HGS/ polyol (HGSIP) and two sub-groups of 50 from these 100 who consumed either sweets containing a very low level of HGS/P or control sweets. Although all subjects received six packets of sweets they were given in completely random order. For each consumption test, subjects received product on day 1, adhered to dietary restrictions (as in the previous study) for 24 h, consumed the product on day 2 and were interviewed on day 3 to record their symptomatology. Therefore subjects consumed one bag of sweets on six occasions, each separated by a wash-out period of one week to avoid acclimatization. The longitudinal study lasted a total of six weeks.
Doses of 50 g, 35 g and 20 g were selected in an attempt to provide HGS/polyol doses above, approximately at and below a hypothetical threshold suggested by the earlier study. Chewy sweets were provided as follows:
- Lycasin® solids (83%) + mannitol (12%) + isomalt (5%) = HGS/P
- Control sweets containing glucose syrup and sucrose = GS/S
To provide subjects with 50, 35, and 20 g HGS/P, bags of sweets contained either 13, 9, or 5 pieces, respectively based on information that each sweet weighed 4.8 g containing 82.1% HGS/P. Hence there were the following sweets available for consumption: HGS/P-50; HGS/P-35; HGS/P-20; GS/S-50.
Consumption was dictated on either an acute or a chronic basis. Acute consumption required that the entire bag of sweets be consumed within 2 h, whereas chronic consumption required that the entire bag be eaten, but that not more than one sweet be eaten in any half hour period during the day.
Table 2 summarises the frequency with which motions were passed as well as the percentage of subjects with diarrhoea (defined in this case as the passage of loose motions on three or more occasions in the 24 h following the consumption of each product). Table 3 gives details of other gastrointestinal symptoms such as bloating, flatulence and colic subjectively ranked using a ten point hedonic scale where 1 to 3 = mild, 4 to 7 = moderate and 8 to 10 = severe. Table 4 shows mean symptom scores with the standard error of the mean for all subjects at the various doses taken acutely and chronically. Figure 2 is derived from this table and shows the average mean symptom score derived from these data.
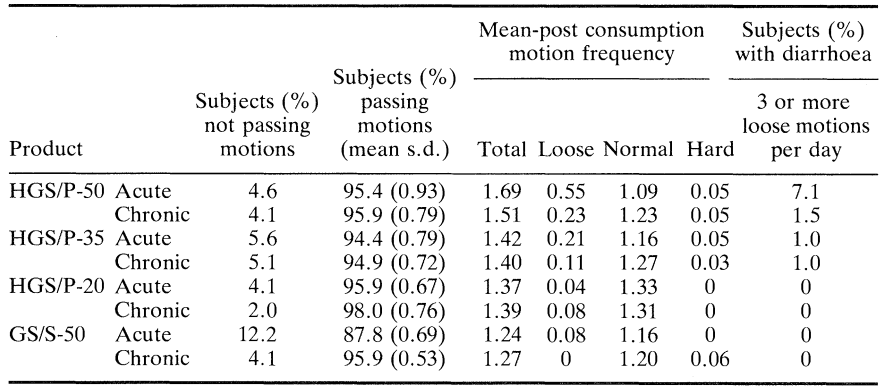
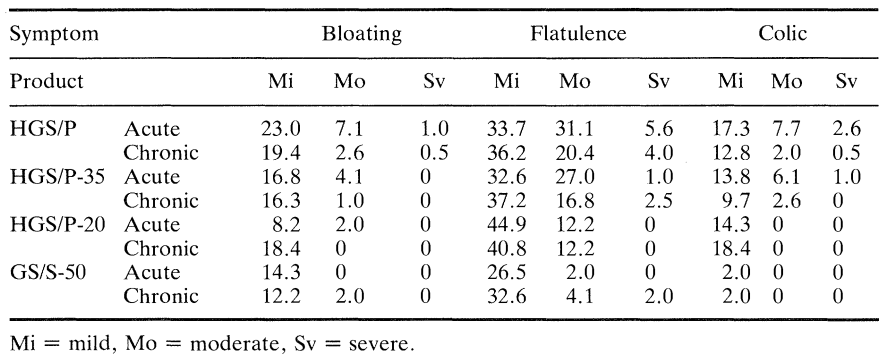
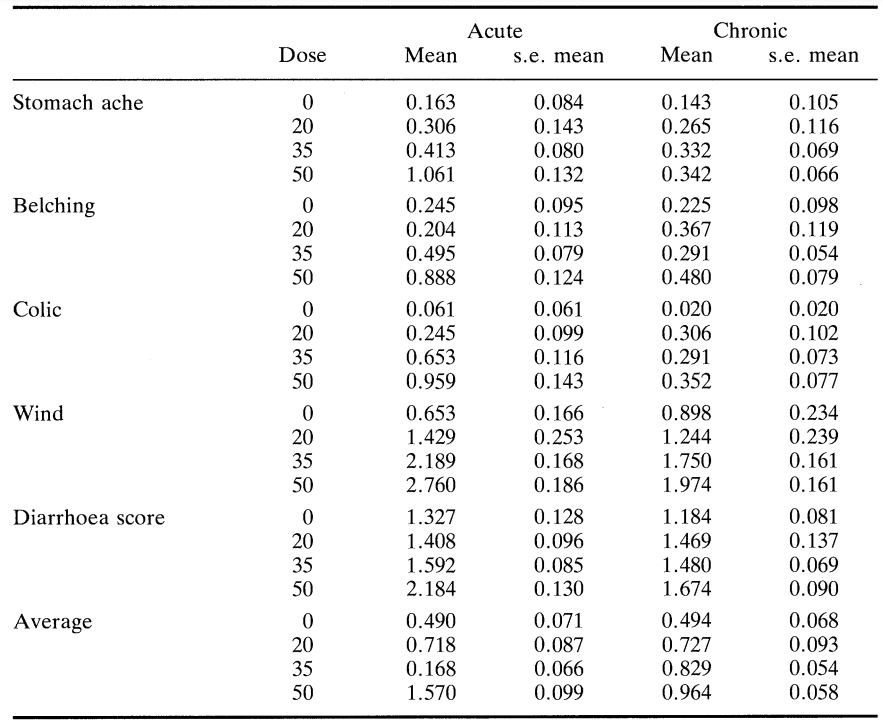
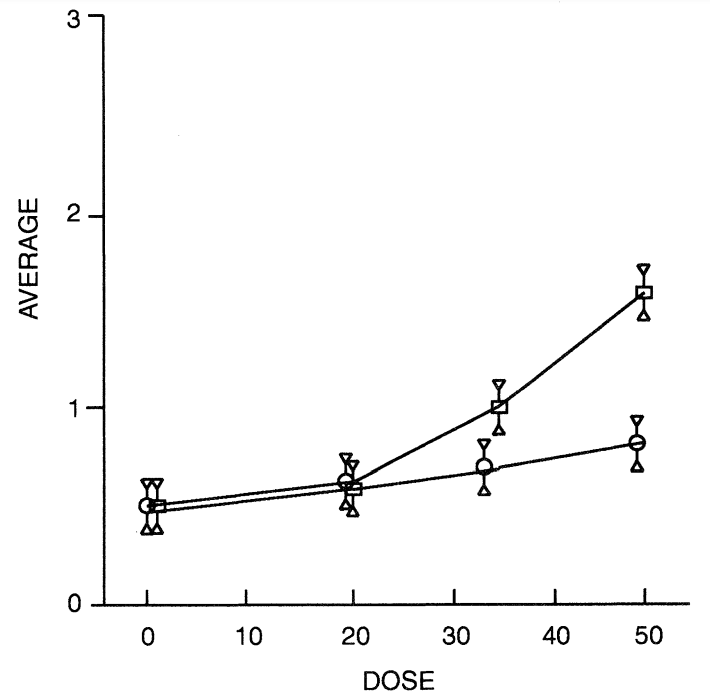
Most subjects passed one normal stool during their post-consumption periods but the passing of loose motions was most closely associated with consumption of the HGS/P-50 and HGS/P-35 sweets. This association was enhanced further by acute consumption. There was a significant increase (P < 0.01) in the occurrence of diarrhoea with increasing dose of HGS/P and this increase was faster than linear for acute consumption but not for chronic consumption. There was also a significant increase in flatulence during both acute and chronic consumption of product as the dose increased. In this case the increased incidence of flatulence was linear in both cases, though greater during acute consumption. Although there was an increase in colic, reporting of this symptom was low for all products irrespective of dose or consumption regime.
A similar study involved using otherwise identical sweets but in this case containing 83% Lycasin solids plus 22% isomalt with the numbers of sweets being controlled to give 50 g and 20 g doses. The same trends were apparent in relation to acute as compared to chronic consumption and also in relation to increasing polyol dose. However, more people provided unprompted information on gastrointestinal symptoms when consuming HGS/P-50 rather than HGS/I-50, but there was no difference in laxativity between the two products.
It may be concluded that consumption of 35 g HGS/P is associated with a tendency towards increased motion frequency and also towards looseness of stools. This tendency is enhanced somewhat if the 35 g dose was ingested in under 2 h or if the dose was increased to 50 g. It would appear that 35 g HGS/P-35 is at, or slightly above the level tolerated by most people but acute consumption tended to enhance the appearance of symptoms in susceptible individuals. Ingestion of 50 g HGS/P exceeded this level of tolerance and a small but increased number of individuals reported intolerance symptoms.
Polyols in chocolate
Maltitol is a widely used sweetening agent because of its physicochemical properties, natural sweetening power (80% that of sucose) and low cariogenicity. Sucrose is generally considered to be cariogenic and standard milk chocolate usually contains sucrose in the range of 40 to 45% as an added ingredient. However, the replacement of sucrose in chocolate by polyols such as maltitol is possible taking into account of a variety of technological properties such as thermal stability, hygroscopicity, sweetness and water content (Zumbe et al., 1994). High purity, crystalline D-maltitol such as Maltisorb® is an excellent substitute for sucrose in sugarless chocolate for technological and organoleptic reasons (Le Bot, 1993; Oliger, 1994; Rapaille et al., 1994; Sicard and Le Bot, 1994).
The gastrointestinal symptoms resulting from eating milk chocolate containing maltitol have been compared with those occurring in the same individuals after eating normal, sucrose-containing chocolate (Storey et al., submitted for publication). In a double-blind crossover study, 20 healthy volunteers (9 males and 11 females between the ages of 18 and 24 years having body mass indices (mean/s.d. of: male 25.1 (3.9) and female 22.0 (1.2)) ingested 100 g bars of milk chocolate which were identical except for their added carbohydrate ingredient. Each 100 g bar contained 40 g bulk sweetener; one product contained sucrose (S), one maltitol (M) and the other a mixture of sucrose and maltitol (SM, 10:30 w/w). Subjects were tested twice with each product given in randomised order determined by a latin square design applied to each leg of the crossover study which related to consumption in fasting and non-fasting conditions. One group consumed S, SM and M during weeks 1 to 3 while fasting and again during weeks 4 to 6 while not fasting. The other crossover group of ten subjects first consumed the products while not fasting and later while fasting. Consumption periods were separated by wash-out periods of 1 week. For 12 h before the test day, the 24 h of the test day and for 12 h thereafter subjects restricted their milk and fruit juice intakes to 300 cm3 each per 24 hand refrained totally from drinking alcohol. Fasting subjects took no food or liquid after 22.00 the night before the chocolate consumption day and consumed the test product as breakfast, being allowed to drink up to 200 cm3 of liquid to ease consumption; a normal meal pattern was resumed from noon on the day of consumption. Non-fasting subjects consumed products within 30 min of the completion of their normal breakfast. Both groups were instructed to eat the entire 100 g chocolate bar within 30 min.
The number of toilet visits made during the 24 h post-consumption was recorded and the consistency of faeces passed on each occasion classified as either watery, normal or hard. Gastrointestinal symptoms were recorded for the 24 h period following consumption as the separate sensations of borborygmi, colic and flatus by ranking them on a hedonic scale where 0 = normal function, 1 = mild symptoms, 2 = moderate symptoms and 3 = severe symptoms. Table 5 gives details of intolerance symptoms and Figure 3 shows the mean symptom score derived from these data.
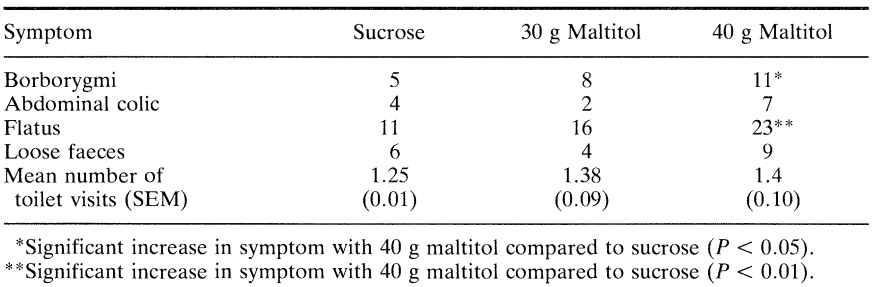
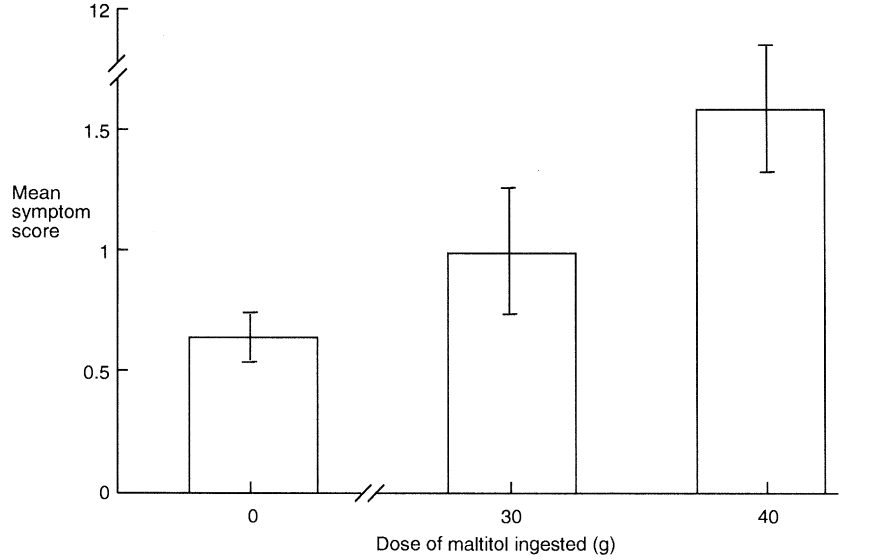
Storey et al. (submitted for publication) discovered that there was no difference in symptomatology between fasting and non-fasting subjects (P > 0.05) nor according to whether maltitol-containing chocolate was eaten before or after normal chocolate (P > 0.05). Eating 30 g maltitol in chocolate caused no increase in gastrointestinal symptoms but ingestion of 40 g increased mild borborygms (P < 0.05) and mild flatus (P < 0.01). Ingestion of 30 g (P = 0.55) or 40 g (P = 0.24) maltitol did not cause any significant increase in laxation; nine subjects passed loose faeces on one occasion after eating 40 g maltitol but this was not significantly greater than after consumption of 30 g maltitol (four subjects) or 40 g sucrose (six subjects). Colic and loose faeces, potentially more distressing symptoms, were uncommon even with the higher dose of 40 g maltitol and the incidence was not significantly higher than after consumption of the 30 g maltitol or sucrose-containing chocolate. More subjects reported multiple symptomatology following consumption of 40 g maltitol (18) and 30 g maltitol (7) compared to sucrose consumption (3; P < 0.01) suggesting that certain individuals are more susceptible than others to incompletely hydrolyzed maltitol and unabsorbed sorbitol entering the large intestine. This may be due in part to intersubject variations in symptom perception and description, but there is little doubt that some individuals appear to be more susceptible than others to the consequences of incompletely hydrolyzed maltitol entering the large intestine along with unabsorbed sorbitol (Dahlqvist and Telenius, 1965; Nilsson and Jagerstad, 1987). These results are consistent with those of Abraham et al. (1981) and Beaugerie et al. (1991) who demonstrated that a 20 to 30 g dose of maltitol syrup could be well tolerated without undesirable symptoms.
These observations of Storey et al. (submitted for publication) that an acute oral intake of 30 g crystalline maltitol in milk chocolate resulted in no significant adverse symptoms and that there was no significant laxative effect when ingested at either 30 g or 40 g per day have major implications for manufacturers of confectionery products. The symptomatology arising from maltitol consumption was not only very low but was also broadly similar in fasting and non-fasting subjects, a consideration of some importance in relation to the propensity of consumers to eat confectionery items as intermeal snacks. It seems that a partitioning of unhydrolyzed maltitol and unabsorbed sorbitol may have occurred in both fasting and non-fasting subjects leading to a similar gradual delivery of such molecules to the large intestine.
Comparative studies of polyols in chocolate
Lactitol and isomalt are two other disaccharide alcohols which have potential as bulk sweeteners for incorporation into confectionery products such as chocolate. There is a considerable literature relating to the appearance of intolerance symptoms following the consumption of these polyols in solution and various other formulations and also information exists relating to possible threshold values below which there is negligible symptomatology. However very few published studies have investigated the ingestion of either lactitol or isomalt incorporated into chocolate. Livesey et al. (1993) found that consumption of lactitol-containing chocolate, isomalt-containing chocolate and polydextrose-containing chocolate resulted in 112%, 73% and 11 % increases in breath H2 respectively compared to sucrose-containing chocolate, this being indicative of a high degree of intestinal fermentation. Broad support was given to these observations by the work of Lee et al. (1994) who found that ingestion of 31.5 g doses of either isomalt or sorbitol incorporated into milk chocolate led to a sharp post-consumption increase in breath H2 excretion compared with that after consumption of an otherwise identical sucrose-containing chocolate. Total breath H2 excretion after eating sorbitol chocolate was significantly greater than after eating isomalt chocolate (P < 0.01). Zumbé and Brinkworth (1992) found that consumption of isomalt-containing chocolate was associated with an increase in motion frequency and flatulence in both normal and Type II diabetics.
Koutsou et al. (1995) have recently compared chocolate containing either isomalt, lactitol or maltitol, three disaccharide alcohols which are increasingly being used as bulk sweeteners in milk chocolate and other chocolate-containing confectionery items such as snack bars. They assessed the effects of ingesting different pol yo Is incorporated into standard milk chocolate by assessing the incidence and severity of gastrointestinal symptoms and comparing them with those occurring in the same individuals after eating normal sucrose-containing chocolate. In a doubleblind crossover study 59 healthy volunteers aged 18 to 24 years ingested 100 g chocolate containing 40 g bulk sweetener as either sucrose, lactitol, isomalt or maltitol or mixtures of sucrose and polyol (10:30 w/w) to give 30 g doses. Table 6 gives details of the intolerance symptoms and shows that 30 g and 40 g lactitol caused a significant increase in flatulence, borborygmi, colic, stool frequency and looseness compared to sucrose (P < 0.01). 40 g isomalt increased all symptoms including mild laxation (P < 0.01) but, unlike lactitol, none was rated as severe. Reduction of isomalt to 30 g was marked by increased tolerance with no increase in stool frequency compared to sucrose (P = 0.35). and evidence of only mild borborygmi (P < 0.01) and mild flatulence, colic and laxation (P < 0.05) 40 g maltitol caused even less intolerance than 40 g isomalt and this was reduced further by a reduction to 30 g. Maltitol had no laxative effect when consumed at either 30 g (P = 0.32) or 40 g (P = 0.13). All these comparisons were made between polyol-containing chocolates and a standard sucrose-containing chocolate bar.
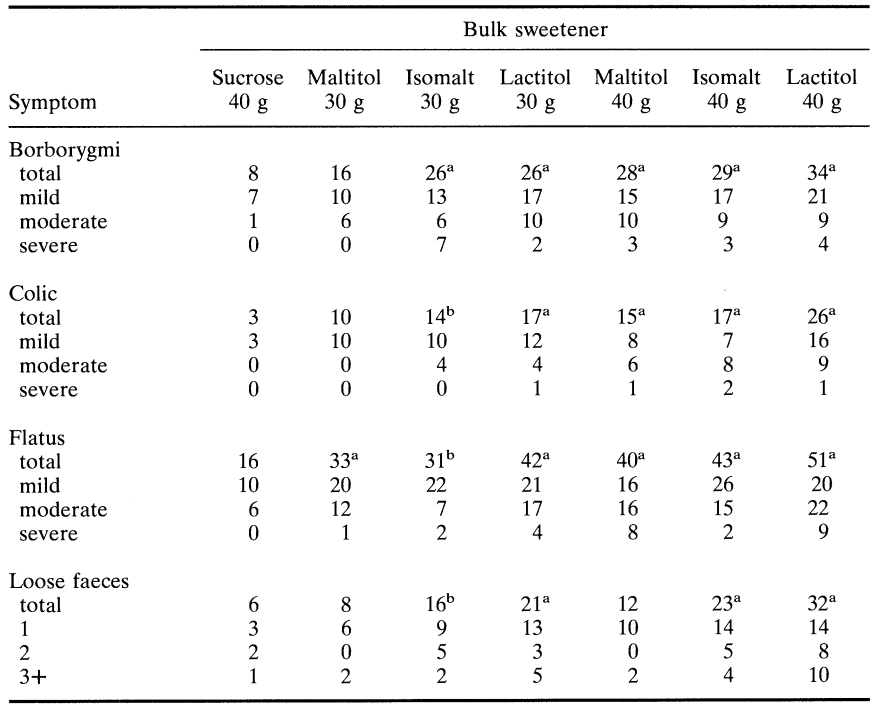
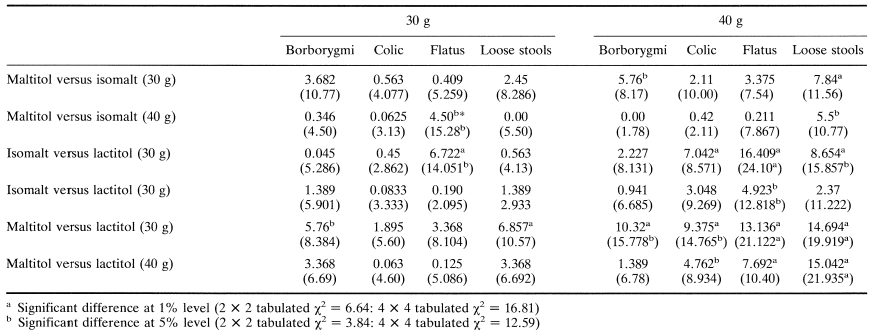
Table 7 compares the symptomatology produced by chocolate containing maltitol, isomalt and lactitol. Very few significant differences were evident between products, most being between lactitol and the other two polyols. However chocolate containing 40 g isomalt produced somewhat looser stools than that containing 40 g maltitol, whereas 40 g maltitol provoked greater flatus than 40 g isomalt. There were no differences in symptomatology between chocolate containing 30 g isomalt and 30 g maltitol.
The relatively low levels of gastrointestinal symptomatology recorded in the studies of Lee et ai. (1994), Koutsou et al. (1995) and Storey et al. (submitted for publication) may relate to the fact that in these studies the polyols were eaten in chocolate rather than in solution or in other non-fat-containing confectionery products such as chewy or boiled sweets. Milk chocolate typically contains approximately 30% fat which, in any digestible form in the presence of bile and pancreatic lipase, is the most powerful agent in the retardation of gastric emptying. Fat digestion products, in particular fatty acids, stimulate the production of cholecystokinin (CCK) by the mucosa of the duodenum and jejunum and CCK then reduces the amplitude of gastric peristalsis which retards gastric emptying. Such an effect is likely to prolong the period of polyol delivery to the small intestine thus ensuring its presentation to the brush border enzymes at a favourable rate.
Nevertheless, the study of Koutsou et al. (1995) confirmed that there are differences between the disaccharide alcohols in terms of their ability to provoke gastrointestinal symptoms. It is likely that this is related primarily to the relative degree of small intestinal hydrolysis and absorption which the polyols undergo. Maltitol undergoes about 10% hydrolysis compared with maltose to yield glucose which is actively absorbed, sorbitol which is passively absorbed (by facilitated diffusion and solvent drag) and residual polyol which enters the large intestine along with unabsorbed sorbitol both of which are then fermented by bacteria, primarily in the proximal colon. Lactitol undergoes less than 3% hydrolysis compared with lactose (Grimble et al., 1988; Metzger et al., 1988; Nilsson and Jagerstad, 1987; Wursch et al., 1989) leaving residual polyol which presumably stimulates laxation because of the high osmotic load in the ileum. Like lactitol, isomalt undergoes marginal hydrolysis in the proximal small intestine (Grupp and Siebert, 1978; Nilsson and Jagerstad, 1987) but the levels of gastrointestinal symptomatology recorded by Koutsou et al. (1995) presumably reflect not only differences in the extent of hydrolysis but also differences in the dynamics of fermentation of the mixture of residual polyol and polyol hydrolysate by the colonic microflora (Livesey et al., 1993) as well as the better absorption of isomalt in the small intestine (Beaugerie et al., 1991; Wursch et al., 1989). Notwithstanding these differences, Koutsou et al. (1995) demonstrated that symptomatology was always dose related and even with lactitol many intolerance symptoms decreased in incidence and severity when the level of ingestion was decreased.
Breath hydrogen excretion
The normal human digestive tract contains about 150 cm3 of gas of which approximately 100 cm3 is in the colon. When unabsorbed carbohydrate enters the colon and is fermented, the total amount and proportion of gases produced depends upon the nature of the fermented residues, but in any case much of the gas is absorbed and excreted across the lung epithelium although some is also consumed by other bacteria. Breath H2 testing has been used by many workers to provide information on the extent and duration of fermentation of unabsorbed dietary components (Bond and Levitt, 1972; Flourie et al., 1988a and b; Fritz et al., 1985; Hyams, 1983; Lee et al., 1994; Oku et al., 1991; Wursch et al., 1989). However, the quantitative aspects of breath H2 excretion must be considered with caution especially when comparisons are to be made because of large inter- and intra-individual differences between individuals (Rumessen et al., 1990) and because of differences in the stoichiometry of H2 production following the consumption of different polyols (Livesey et al., 1993). Hammer (1993) demonstrated that colonic H2 absorption is highly effective at low colonic hydrogen accumulation rates (i.e. less than 76 cm3 H2/6 h) but not so at higher concentrations when absorption can decrease to as little as 20%. Nevertheless, interindividual differences in breath H2 excretion after the ingestion of a standard dose of carbohydrate (e.g. 12.5 g lactulose) are probably due to differences in bacterial net gas production rather than differences in gas absorption. That is, incomplete colonic gas absorption is the consequence, and not the cause, of high colonic gas accumulation rates.
Notwithstanding these difficulties in the interpretation of breath H2 excretion, the technique has a certain attractiveness because it is non-invasive and does not involve the use of radiolabelled molecules. Furthermore, other available techniques suffer from many of the same limitations. Storey et al. (submitted for publication) have used the breath hydrogen test to assess the colonic fermentation of polyols ingested in milk chocolate rather than in liquid form and have shown that the ingestion of milk chocolate containing maltitol leads to an elevated breath H2 response compared with that following the consumption of milk chocolate containing only sucrose, a difference which is significant at the 1% level and is indicative of a greater degree of intestinal gaseousness due to bacterial fermentation of maltitol and sorbitol. Non-adapted, fasting subjects were tested with chocolate product in a randomized order with test periods separated by 1 week. Test products were consumed in 30 min immediately prior to breath testing and the positive control was a 20 ml dose of lactulose syrup. A sharp rise in breath H2 concentrations after polyol consumption in chocolate marked the beginning of colonic fermentation, effectively the orocaecal transit time, being 115.5 min. (± 18.7) for milk chocolate containing 30 g maltitol and 87.0 min (± 14.3) for a similar product containing 40 g maltitol. Ingestion of 40 g maltitol in chocolate led to a greater total H2 excretion compared with that after eating chocolate containing either 30 g maltitol (P < 0.05) or sucrose (P < 0.01). In a similar study Lee et al. (1994) showed that breath H2 excretion after consumption of chocolate containing either sorbitol or maltitol was significantly higher than after consumption of chocolate containing sucrose (P < 0.01). The dose-related breath H2 response observed by Storey et al. (submitted for publication) was consistent with a lower reported symptomatology following ingestion of 30 g maltitol compared with 40 g maltitol. The total breath H2 excretion after lactulose was 183.1 ppm ± 40.8 and, taking this as 100%, it was calculated that on average, 34.7% (10.4 ± 1.4) of a 30 g ingested dose and 49.2% (19.7 g ± 4.0) of a 40 g ingested dose of maltitol was fermented in the colon. An estimated maltitol hydrolysis and constituent sugar absorption of ca. 65% of a 30 g dose and ca. 50% of a 40 g dose is in broad agreement with the 50% malabsorption of maltitol reported by Wursch et al. (1989) and Beaugerie et al. (1991).
The dynamics of the gastrointestinal response to the ingestion of hydrogenated glucose syrups and polyols depend upon many factors including (i) the extent of digestion of the native polyol, (ii) the extent of absorption of the products of hydrolysis, (iii) the inherent fermentability of the residual polyol and its hydrolysis products, (iv) the fermentative capacity of the particular colon under consideration, (v) the extent of colonic absorption of fermentation products, (vi) the osmotic properties of the polyoIlhydroJysis products mixture, (vii) the ‘sensitivity’ of the large bowel muscularis externa. One aspect of the work of Lee et al. (1994) and Storey et at. (submitted for publication) concerning the ingestion of polyols incorporated into milk chocolate was to attempt to correlate the breath hydrogen response (BRR) with the incidence of gastrointestinal symptomatology in non-intubated subjects thus taking all the above considerations into account. Livesey et at. (1993) pointed out that the interpretation of the BRR is confounded by the differing stoichiometries for hydrogen production, by interaction between substrates and by uncertainty concerning the extent to which small intestinal hydrolysis yields species with a stoichiometry that differs from the parent substrate. Nevertheless, although the interpretation of BRR is obviously complex and must be made with circumspection, there seems to be little doubt that, taken together with information relating to the incidence of intolerance symptoms in the individuals providing the breath samples, it does give a useful insight into the events occurring in the intact gut concerning the degree of digestion and fermentation of ingested polyol.
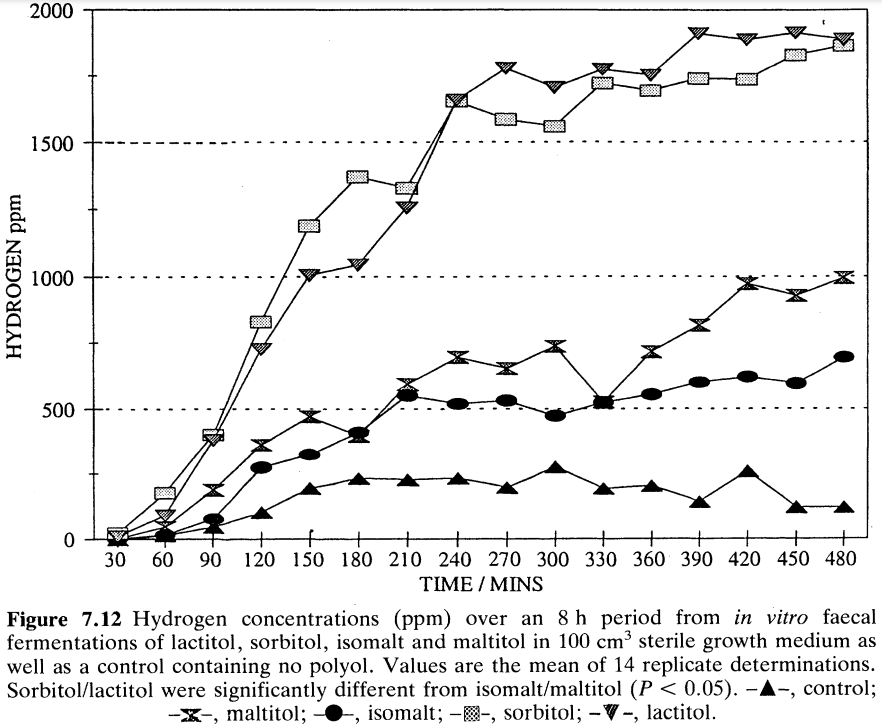
Livesey et al. (1993) refer to the low production of H2 from isomalt compared to other polyols and polyol mixtures after in vitro faecal fermentation. We have also performed such studies and one relevant data set is presented in Figure 4 which confirms that crystalline maltitol as well as isomalt appear to be inherently less fermentable than some other sugar alcohols such as sorbitol and lactitol. It must be borne in mind, however, that the confines of an inert in vitro fermentation vessel cannot replicate exactly the conditions within a human colon and also, as yet, there is no way of obtaining a standardized faecal inoculum. In vitro faecal fermentation is a useful technique, but much remains to be defined in terms of its suitability to predict with confidence what might happen in the colon of a person ingesting polyol. Like breath hydrogen monitoring, it should be used with caution along with data from consumption studies to explain the gastrointestinal response to polyols and mixtures containing polyols (Storey et at., 1994).