The glucose isomerases used are immobilized and granulated to a particle size between 0.3 and 1.0 mm. Mg2+ acts as activator and stabilizer of the enzyme, and is therefore added to the feed syrups in the form of MgS04 ·7H20. The amount necessary depends on the presence of calcium ion, which acts as an inhibitor in the system by displacing the magnesium ion activator from the isomerase molecule. Therefore the Ca2+ ion content should be kept as low as possible. At a Ca2+ ion content in the feed syrup of 1 ppm or lower, the addition of 45 ppm Mg2+ (e.g. approximately 0.6 g of MgS04 ·7H20 per 1) will be sufficient. At higher concentrations of Ca2+ ion, a proportionate weight ratio between Mg2+ and Ca2+ ions should be provided.
Each reactor load of Sweetzyme is used for a long period of time, thereby being exposed to a very large quantity of syrup. In consequence, the accumulated effect of even low levels of food syrup impurities may lead to significant reduction in the enzyme lifetime. Some impurities may chemically inactivate the isomerase, whereas others may adsorb on to the isomerase particles, gradually blocking their active surface. In order to attain maximum enzyme lifetime, it is therefore very important to purify the feed syrup (including possible recycle streams) thoroughly before isomerization:
- The raw high-DX liquor from saccharification is filtered to remove particulate material which might clog the isomerase particles. Soluble impurities (peptides, amino acids, ash etc.) are potential inhibitors of the isomerase and must therefore be removed by carbon treatment and ion exchange. The efficiency of the carbon and ion exchange treatments should be checked by measuring the UV absorbance and conductivity of the purified feed syrup. •
- Dissolved oxygen in the feed syrup increases byproduct formation, and some byproducts may inhibit the enzyme. Therefore, the oxygen content of the syrup should be minimized by vacuum deaeration after heating to isomerization temperature. Sulphite can be used as an oxygen scavenger, but should not be used in amounts exceeding approximately 100 ppm S02 in the feed syrup.
- Before entering the isomerization reactor the syrup should be passed through a 5-10 [,lm check filter.
- Some impurities (e.g. maltulose) which are difficult to remove by the above mentioned procedures may have been formed during the starch liquefaction process. This byproduct is minimized by avoiding excessive exposure of the gelatinized starch to high temperature and pH.
The main criteria for selecting the feed syrup specifications are optimization of enzyme productivity and limitation of byproduct formation. Typical feed syrup specifications are shown in Table 2.10.
| Temperature | 55-60°C |
| pH | 7.5-8.0 |
| Dry substance content | 40-50 wt% |
| Glucose content | ≥95% |
| S02 | 0-100 ppm |
| Calcium ion | ≤ 1 ppm |
| MgS04 ·7H20 (activator) | 0.15-0.75 g l-1 |
| Conductivity | ≤ 100 μS cm-1 |
| UV absorbance (2S0 nm) | ≤ 0.5 |
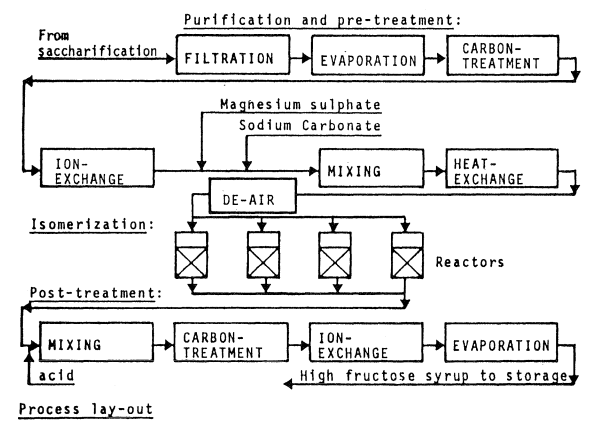
Process layout
Figure 2.23 is an example of process layout covering purification, isomerization and syrup product finishing.
Enzyme decay
During operation the immobilized enzyme loses activity. Most commercial enzymes show an exponential decay as a function of time as shown for SwQ in Figure 2.24, but with SwT (Sweetzyme T is the Streptomyces murinus glucose isomerase from Novo Nordisk) a linear activity decay is found.
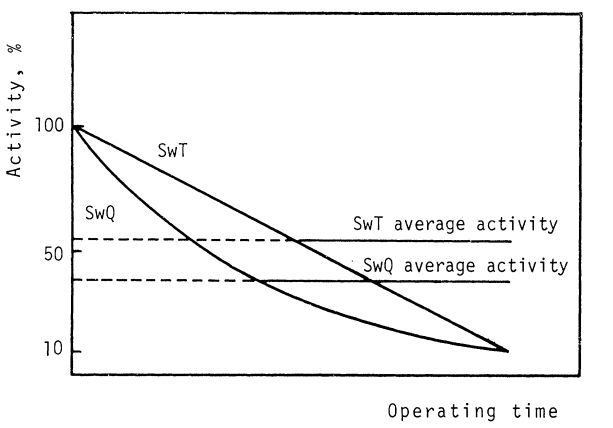
Typically, a reactor load of glucose isomerase is replaced after three half lives, i.e. when the activity has dropped to around 12.5% of the initial value. The most stable commercial glucose isomerases have half lives of around 200 days in industrial practice.
To maintain a constant fructose concentration in the product syrup, the feed flow rate is adjusted according to the actual activity of the enzyme. With only one isomerization reactor in operation, excessive variations in syrup production rate would be the result. To avoid this, several reactors containing enzymes of different ages are operated in combination.
Reactor design for glucose isomerization in the United States has been described (Blanchard and Geiger, 1984). Reactor diameters are normally between 0.6 and 1.5 m. Typical bed heights are 2-5 m. Minimum bed height:diameter ratio for one reactor is 3:1 to ensure good flow distribution. Plants producing more than 1000 t of HFCS (based on dry matter) per day use at least 20 individual reactors.
