This article discusses the commercial use of glucose syrups in industrial fermentation processes in the United Kingdom. It is split into two sections, the first covering the use of glucose syrups in the production of food ingredients, processing aids, chemicals, and pharmaceuticals. The second part covers the history and use of glucose syrups in the United Kingdom brewing industry. Glucose syrups are used widely in the fermentation industries in the United Kingdom. Each year over 100000 tonnes of syrups are sold into areas of the fermentation industry including brewing, pharmaceuticals, chemicals, cider, wine and sherry, novel foods, and gums. This represents a significant percentage of the total UK glucose syrup usage.
Introduction
Glucose syrups are products of the hydrolysis of starch into mixtures of D-glucose polymers. The most common glucose syrup described here is a product composed almost entirely of dextrose and has a Dextrose Equivalent (DE) of 90 or more. This provides the most readily usable form of fermentable sugar for the processes described herein. It should be noted that such highly converted glucose syrups are often referred to as hydrosylates and the term is used synonymously in the industry.
Glucose syrups of 95 DE are produced by the enzymic hydrolysis of starch, using carbohydrases such as a-amylase and amyloglucosidase to break down the starch source predominantly to dextrose. Other enzymes can be used to produce different carbohydrate spectra according to the needs of the end user, such as the maltose-rich syrups commonly used in the brewing industry. Examples of the range of products available are shown in Table 1.
| Sugar | 20 DE | 42 DE | 63 DE | 95 DE | High maltose | Very high maltose |
| Dextrose | 1.5 | 19 | 37 | 92 | 3 | 3 |
| Maltose | 7.0 | 14 | 32 | 5 | 55 | 70 |
| Maltotriose | 11.0 | 11 | 12 | 3 | 15 | 20 |
| Higher sugars | 56.0 | 56 | 19 | 0 | 27 | 7 |
Note that some of the industrial processes discussed below have a high proportion of freely available information on the process involved and the advantages of the types of glucose syrups used. Other industries consider this information proprietary and the degree of depth of each section will reflect this.
The history of glucose syrups has been discussed in many previous books, but it is worth noting that by 1870 a large industry was already established in Germany and France for the production of glucose syrups (Mulvihill, 1992), the applications of which included wine manufacture and brewing. By the 1920s, progress in dextrose crystallization had made available anhydrous and monohydrate forms at prices competitive with sucrose. The production of glucose had become a large and stable industry.
More recently major advances in enzyme and process technology have allowed glucose syrup manufacturers to produce glucose syrups which can be tailored to specific customer needs, whether they are for very high dextrose, high maltose or fully soluble low conversion syrups to suit the specific needs of the organism or process involved.
Production of food ingredients and additives
Gums
Traditionally gums (polysaccharides) are used in the food industry to provide a wide range of functional benefits such as thickening and gelling. Natural gums such as acacia gum or gum tragacanth are extracted from plants. The seasonal fluctuations in price and supply of these have made the advent of industrially produced fermentation products such as xanthan gum and gellan gum attractive alternatives. Often blends of gums are used to achieve the desired properties. For instance, xanthan forms a thermoreversible cohesive gel system with locust bean gum.
Xanthan gum is a mixed polysaccharide with a remarkable resistance to heat and pH changes. It is produced on a wide scale in the world from the fermentation of glucose syrup. The type of glucose syrup used will depend on the specificity of the strain of Xanthomonas campestris used, and the availability and price of local glucose syrups. In the USA 42 DE syrups have traditionally been used as the most cost effective carbon source. It is known that both 63 DE and high fructose syrups can be used, again dependent on the supply economics and gum yields involved. Syrups used need to be low in color to ensure the final product is low in color itself, so substrates such as molasses would not normally be suitable.
Xanthan gum is produced by aerobic submerged fermentation (Kelco, 1994). The fermentation medium contains glucose syrup, a nitrogen source, and trace elements. It is a batch process where the key parameters such as pH, temperature, and aeration and agitation are closely controlled. On completion of this stage the broth is pasteurized and the gum is recovered by precipitation through the addition of propan-2-ol. The alcohol is then removed and the product is dried, milled and sieved to a specified particle size, tested and packaged.
Novel foods
Novel foods (ICI, undated) derived from fermentation have had a rather chequered history. Products such as protein derived from methanol/ ammonia fermentations gave a great deal of experience to the design and running of continuous fermenters, but were a mixed success commercially.
More recently the advent of mycoprotein on to the UK market has shown signs of promise. Mycoprotein is a protein and fiber-rich mycelial food produced by the continuous fermentation of a selected strain of Fusarium graminearum on a high DE glucose substrate. Note that the steps from sterilization through to RNA reduction are carried out under conditions of sterility.
Following RNA reduction and filtration mycoprotein emerges as a filter cake of about 30% solids, which is subsequently texturized and flavored to make a range of meat substitutes. The type of glucose syrup needed for the process is a deionized 95 DE syrup. As the process is sensitive to changes in substrate, generally fermentations are restricted to one source of supply to maintain high yields. Fermentations can run for 500 to 1000 hours before a morphological change occurs which renders the final product incapable of being texturised. The process then has to be shut down, the plant cleaned and sterilised, and restarted. Deionised glucose syrups are needed for the following reasons
- The mycoprotein is only filtered to remove water, thus any substrate characteristics such as color would be likely to transfer over to the finished product.
- The mycoprotein has a very high flavor absorbency, and thus can pick up flavor notes from the substrate.
- High chloride levels in a syrup can give rise to stress corrosion in stainless steel fermenters over a period of time.
Chemicals
Glucose syrups have long been used in fermentations to produce acidulants, such as citric acid (Haarman and Reimer, undated), fumaric acid, propionic acid and lactic acid (Rhodes and Fletcher, 1966). Citric acid is by far the largest in commercial terms and is the product covered in most detail here. Gluconic acid is also produced commercially.
Lactic acid
Lactic acid is made by either chemical synthesis or by fermentation of glucose. In the fermentation process glycolysis of the glucose syrup (preferably 95 DE, concentration 5-20% w/v) is carried out by the organism to produce pyruvic acid, which under anaerobic conditions is broken down to produce lactic acid. The organism is a bacterium such as S. lactis and is microaerobic. The fermentation takes place at 40-50°C over about 2-3 days and needs no aeration. The pH is continuously controlled to counteract the acidity of the lactic acid. Yields of 90% from the 95 DE syrup used are commonly achieved. Contamination by other organisms is not usually a problem as high temperature and lack of oxygen prevent this. Separation then takes place to produce a material suitable for use in sauces, pickles, drinks, etc. It is also used in detergents as a substitute for polyphosphates, and in other cleaning products.
Citric acid
Citric acid is one of the most widely used food acidulants. Initially it was extracted from natural sources, such as lemons. Demand outstripped supply up until the 1920s, when the large scale commercial production of citric acid by Aspergillus niger was developed.
Citric acid is an essential ingredient in soft drinks, food, confectionery and pharmaceuticals. It is produced in two basic forms, anhydrous and monohydrate.
Initially the surface process was used, where the A. niger is grown on trays containing media of sterilised beet molasses and inorganic salts housed in ventilated rooms. A sterile air stream supplies oxygen and simultaneously cools the fermentation. The mould forms a mycelium layer on the liquid surface of the flat trays, where after around 10 days, the mycelium layer is removed and the citric acid is precipitated as calcium citrate. This is then filtered and washed, regenerated as citric acid, decolorised, concentrated, dried, sieved and packed. A significant proportion of the world’s citric production is still made by this method.
After the Second World War submerged fermentation processes were developed and today 80% of world production is made by this type of process which is carried out as an aerobic fermentation in tower fermenters. It is favoured owing to:
- its ability to use a wide range of sugar containing raw materials
- higher yield of citric acid
- improved process control and shorter time
- lower labour and space costs
- better control of sterility
Separation of the citric acid is similar to the surface process. Raw materials for the submerged citric acid production process include sucrose, raw sugar, starch, glucose syrups, beet molasses and maize grits. Choice of raw material generally depends on local economic factors. Exceptions to this occur where certain advantages can be gained through the use of purer raw materials, for example partial substitution of a relatively impure substrate such as molasses by glucose syrup to increase output, reduce recovery and purification costs and lower the effluent loading. The most popular type of glucose syrup is a 95 DE product, where maximum fermentability is needed to ensure maximum yield. Citric acid producers are known to purchase raw starch and convert this on site to a 95 DE product. A fully deionised and filtered syrup is not needed for this fermentation, . so a partially filtered glucose syrup is particularly cost effective.
Ethanol
Ethanol has been produced from fermentation processes for thousands of years. Most ethanol for use as a chemical feedstock is produced by chemical means, primarily through the catalytic hydration of ethylene. In the light of concern over energy shortages, the production of ethanol from carbohydrates such as glucose syrup has been studied extensively. Under anaerobic fermentation conditions and high glucose concentrations S. cerevisiae produces ethanol, once optimum biomass concentrations have been produced under aerobic conditions. Many different sources of carbohydrate can be used, however 95 DE glucose is the most common form of glucose syrup in use. Degree of refining of the carbohydrate substrate is not normally important, as long as it is of a consistent quality.
Gluconic acid
Gluconic acid is produced from glucose syrup by microbial oxidation via Glucose oxidase. High DE syrups are used to produce the gluconic acid via a submerged fermentation process using Aspergillus niger or Acetobacter suboxydans. Gluconic acid is used in the manufacture of leather and foods, and also in the processing of certain metals.
L-Ascorbic acid
The production of L-ascorbic acid (vitamin C) consists of several chemical steps and one microbial conversion. The oxidation step from D-sorbitol to L-sorbose is carried out by Acetobacter suboxydans in a submerged process at 30-35°C with aeration and agitation. Deionised 95 DE glucose syrup is used as the raw material for the process.
Pharmaceutical products
Many pharmaceutical products are derived from fermentations based on glucose syrups, such as penicillin, cephalosporin and griseofulvin. Worldwide in this application the consumption amounts to over 150,000 tonnes of glucose syrup per year. The substrate generally used is a 95 DE syrup, as most of the organisms use only the dextrose fraction of the syrup, so it must have as high DE as practicable. Deionisation is not always necessary as salts and growth factors are added to the fermentation. The colour of the syrup is important where a light coloured end product is required.
Certain intermediate products to be grafted onto traditional antibiotics such as penicillin are fermented from very low DE syrups. This is due to a soil-based organism being used which prefers high molecular weight substrates naturally found in its normal habitat. The type of glucose used is typically a 20 DE product with a high proportion of oligosaccharides.
Griseofulvin
Griseofulvin is a non-toxic systemic antifungal antibiotic for the treatment of fungal diseases by oral therapy, such as ringworm. It is produced by submerged fermentation using a strain of Penecillium griseofulvin. A 95 DE glucose syrup is used as the carbohydrate source and is fed into the medium on an intermittent basis. Good aeration is necessary, along with careful control of available nitrogen, pH and the concentrations of phosphate and calcium carbonate. Addition of the glucose syrup causes the pH to rise as consumption of the glucose takes place, but this can be controlled by the addition of more carbohydrate.
Growth promoters
Growth promoters for the animal feed industry can be produced from a fermentation process using 95 DE glucose syrup as the main carbohydrate source. Once the fermentation is complete, the broth is spray dried, packed and sold. The growth promoters are believed to encourage the growth of acid bacteria in the animal gut and slow the growth of the methane producing bacteria thereby enhancing growth rates of the animal.
Enzymes
Enzyme production by fermentation constitutes a huge market throughout the world, with high growth rates. Around half the enzyme production in the West is centred in Denmark. Most enzymes are hydrolytic in action and are used for the depolymerisation of natural products. Many are proteolytic, for use in the dairy, detergent and leather industries. The baking, brewing, distilling, textile and starch industries all use considerable quantities of carbohydrases. Little information is freely available on the fermentation processes used in the manufacture of enzymes, but it is known that 95 DE syrups, usually deionised, are used as the carbohydrate substrate for many fermentations.
Use of syrups in brewing
The earliest records of brewing date back to Egyptian times, over 4000 years ago. The original beverages were made from cereals, which were probably allowed to germinate in order to produce the enzymes necessary to hydrolyse the starch to fermentable sugar before addition of the yeast to convert the sugars into alcohol.
Introduction
By the Middle Ages the process of brewing had progressed so that the grain was germinated and dried to produce malt, which could then be crushed and mixed with water at a suitable temperature (ca. 65°C), to produce a mash allowing the starch from the grain, usually barley, to be broken down into sugars prior to fermentation. It was found that other carbohydrate sources which were readily available and often cheaper as a source of extract (fermentable carbohydrate) could be used to augment or replace some of the malt.
Adjuncts (unmalted carbohydrates) can be generally classified into solid adjuncts, usually other cereals which are a pure source of starch and use the enzymic capacity of the malt to convert the starch to sugars. It is often necessary to cook or boil the starch in a separate vessel to gelatinise it before blending it with the malt mash to complete the saccharification or conversion of the starch to fermentable sugars. Alternatively sugars and syrups can be used which come either directly from sugar sources such as honey, cane or beet, or are produced by a sugar refiner from the external hydrolysis of cereal starches with acid or enzymes. For many centuries the only available source of sugar was honey, which is still used to produce its own fermented beverage, mead, as well as speciality beers such as Bragot’s ale. It was also used to sweeten beers. In the nineteenth century treacle and molasses were used to produce dark rich full flavoured ales, and it is not until the twentieth century that clearer, lighter, more purified sugars and syrups became available.
As well as a source of additional fermentable extract, sugars contribute other properties, which give the essential character to certain styles of beer. For example many milds and sweet stouts contain caramel (burnt sugar) for both flavour and colour. Sugars can be added at the end of primary fermentation to provide sweetness or additional extract to promote a secondary fermentation.
In addition to the amount of adjunct used, there is an interesting difference between the types of adjunct favoured in different parts of the world. Most American and European brewers use solid non-gelatinised adjuncts, whilst in the UK and Australia sugar or syrup adjuncts are preferred. This may be partially explained by the availability of the different raw materials, but it is also a consequence of history and legislation and has had a major influence on the development of brewing in different countries.
History of the use of adjuncts
Adjuncts have not always been permitted in the brewing of beer, especially as brewing has consistently been exploited as a source of revenue for governments. A traditional method of raising taxes both in the UK and Germany was on malt, and hence there was strict legislation prohibiting brewers from using adjuncts to evade duty when taxation was based on the weight of malt used.
The best known legislation is the German ‘purity law’ or ‘Reinheitsgebot’ , first introduced in Munich in 1487 by Duke Albrecht IV and extended to Bavaria in 1516 by Duke William IV at the Diet of Ingoldstadt, from where it spread to cover most of Germany. As well as securing revenue, the purity laws were considered a guarantee of quality.
It is probably less well known that the United Kingdom had its own purity laws. Malt was first taxed in 1660 in the reign of Charles II, but it was in the eighteenth century that legislation prohibiting the use of brewing materials other than malt and hops was introduced. These laws remained in place until the early nineteenth century when poor barley harvests and the availability of sugar imports allowed a temporary relaxation in the law.
In 1847 the law was repealed to allow up to 25% of the grist (fermentable extract) composition to be made up by sugar on a permanent basis. The principle reason was to put the sugar cane grown in the colonies on the same footing as home grown barley. The sugar was taxed in a similar manner to malt. However, the repeal of the purity law was followed by a strong lobby by the barley growers for its reinstatement, with opposition from the brewers who formed a ‘Free Mash Tun Association’, perhaps the forerunner of the Brewers Society.
The arguments were brought to an end in 1880, when the Free Mash Tun Act was passed repealing the duty on malt and imposing duty on the volume and specific gravity before fermentation. To quote Mr Gladstone’s own words, “The brewer will brew from what he pleases and have a perfect choice of his material and his methods”.
The replacement of malt with sugar and the use of sugar for priming was welcomed at the time since it enabled brewers to reduce fermentation time as well as improving the general quality of the beer. Furthermore, the large excise levies on the specific gravity of fermenting beer had a profound effect on the development of British brewing. Brewers could lower costs by reducing beer losses which carried excise duty. They also preferred to fully ferment beers to produce the maximum alcohol from the sugars present. This encouraged brewers to produce beers with lower final specific gravities and to use highly fermentable syrups to produce beers with low residual sugars to minimise duty charges.
In 1993 the excise rules changed again with excise now being raised on the percentage alcohol and the volume of beer sent out of the brewery gate. Potentially this could have as big an effect as Mr. Gladstone’s ‘Free Mash Tun’ legislation, allowing brewers to adjust the gravity and sweetness of the beer without suffering an increase in duty unless it produced additional fermentation. As a further consequence priming sugars may find a new lease of life with the production of lower alcohol beers with more body through higher residual sugar levels.
Why the British favoured sugars and syrups
Whilst most other countries use cereal adjuncts such as maize, rice or wheat, the UK has chosen to use syrups made either from a starch source such as maize or wheat or as a refined syrup from cane. Why the British brewers preferred sugar is not known, but is probably due to a combination of factors, some of which are considered here.
The British generally had higher quality malts which enabled them to use single temperature mashing in mash tuns (combined malt conversion and filtration vessel) as opposed to mash kettles (multi-temperature conversion vessels) and lauter tuns (separation vessels) principally used in Europe and North America. This meant they did not need the cereal cookers available to them to use non-gelatinised starch sources.
Currently Britain still tends to use indigenous two row barley varieties, whilst Europe and America use quite a high proportion of six row barley. The six row barleys tend to produce malt with higher enzymic power thus giving them greater potential to saccharify cereal adjuncts, which in North America can be as high as 40% to 50% of the goods.
As well as the difference in enzymic power, Britain tends to produce more dark beers such as ales and stouts. Ale malts due to their higher curing temperatures have a lower enzymic power when compared with lager malts made from similar two row barley. The lower ale malt enzymic power will serve to reduce further the opportunity for solid adjuncts in the mash tun.
Use of syrups in brewing
Until the 1970s around 70% to 80% of beers produced in the UK were ales or stouts, and these beers have a more robust flavour and are less likely to be affected by other flavours introduced from the semi-refined cane syrups. Some of the syrup flavours are described as ‘luscious’ and help to give the beer a unique fullness and flavour. With darker beers the colour of the syrup was unimportant, since it could be compensated by the grist composition. Sugars and caramels are used to enhance and adjust colours. Until the 1970s major brewers were individually colouring cask beers for particular customers and this practice may still continue among some of the smaller brewing companies.
Britain is the principal producer of cask conditioned beer, which requires a secondary fermentation in the cask to produce carbon dioxide before the beer is ready for consumption. During fermentation the malt sugars are taken up in a very specific order with sugars such as glucose and sucrose taken up first, followed by maltose and then maltotriose, so that by the end of a regular fermentation only a few slowly metabolisable sugars remain to generate the secondary fermentation. Brewers in the 1840s found that adding cane priming sugars (sucrose) speeded up the secondary conditioning and made the beer ready for trade more quickly.
Syrups also have a considerable benefit in that they can be added directly to the wort kettle, and hence supply fermentable sugars without using mash tun capacity. This can increase the brewhouse throughput without the need for additional capital investment. Because syrups have a very high specific gravity, they can be used to increase the wort kettle gravity, which has a number of practical benefits to the brewer:
- More fermentable sugars can be extracted from the mashing process, thus improving yield without prejudicing the wort kettle specific gravity.
- Traditionally high gravity beers such as barley wines, which previously had to be boiled for several hours to achieve the wort kettle specific gravity, could have their boiling times reduced by making up the gravity with syrup.
- The use of syrup to increase gravity enabled the UK to develop high gravity brewing, with the benefits of increased capacity without additional capital investment or loss in brewhouse yield.
Designing syrups to meet the brewer’s needs
The use of acid hydrolysis of starches to produce crude chip glucose was not readily available until the 1840s, when a typical syrup would contain up to 40% glucose, the balance being made up of higher sugars. As starch enzyme technology developed from the 1920s onwards syrup manufacturers were able to control the hydrolysis of the starch molecule and today are able to produce a complete range of different sugar spectra.
It was not until the 1960s which brought the need to brew more delicately flavoured products such as lagers, that starch-based syrups were more widely used. However, the range of products from highly fermentable glucose syrups with a similar ferment ability to all malt derived beers have encouraged brewers to use syrups as a way of controlling the fermentability of their beers.
| Sugar | Wort | 63 DE | Maltose | VHM* | Dextrose | Maltodextrin |
| Dextrose (%) | 13 | 38 | 2 | 3 | 94 | 0.2 |
| Maltose (%) | 52 | 33 | 55 | 71 | 3.5 | 1.5 |
| Maltotriose (%) | 12 | 6 | 16 | 16 | Trace | 3.5 |
| Higher sugars (%) | 23 | 23 | 27 | 10 | 2.5 | 94.8 |
| Fermentability (%) | 71 | 74 | 65 | 95 | 98 | 4 |
| Extract | 270 | 300 | 310 | 256 |
Table 2 shows examples of the range of carbohydrate composition of selected starch-based syrups with a breakdown into monosaccharides (DP1), disaccharides (DP2), trisaccharides (DP3) and dextrins (DPn).
Syrups have traditionally been added directly to the wort kettle where they behave as follows:
- Contribute flavour and aroma to beer either through their own character or by diluting those flavours contributed by other grist components such as the malt.
- Improve beer stability by diluting the non-starch constituents of wort such as proteins and polyphenols which contribute to beer haze.
- Subject to fluctuations in malt prices they can often represent a cheaper source of fermentable sugars.
- Extend the brewhouse capacity by increasing throughput without capital investment.
- Add directly fermentable sugars for better brewhouse efficiency and high gravity brewing.
- Depending on the syrup used it may either enhance or dilute wort and beer colours.
- Syrups can also reduce the concentration of wort nutrients such as nitrogen, amino acids and minerals. Whilst a high adjunct level can restrict fermentation and possibly reduce the amount of foam-forming compounds, lower nutrient levels help to reduce ester formation. A lower wort nitrogen-to-carbon ratio produces lower esters, thus adjuncts can be used in high gravity brewing to avoid overproduction of esters.
- Different syrups have different fermentabilities which may be used to control the fermentability of a beer.
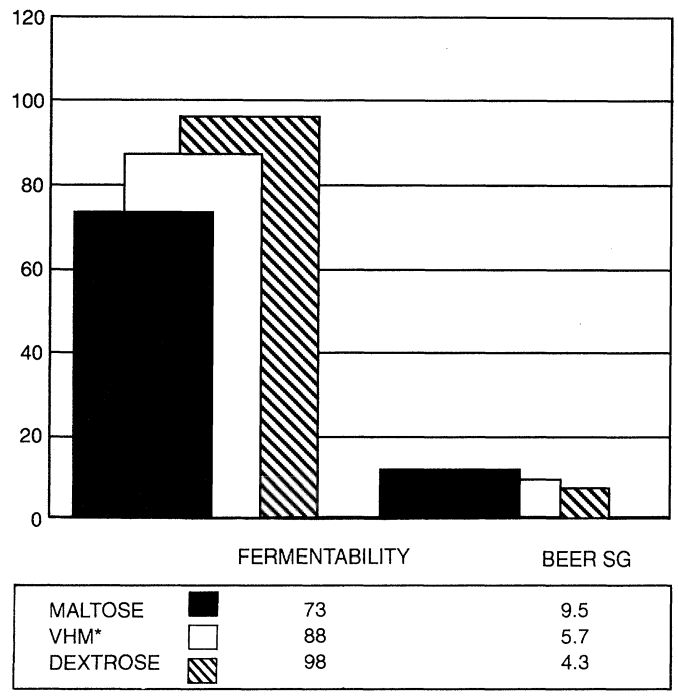
The way in which syrups can be used to control the fermentability of a beer is shown in Figure 1 where 60% of the fermentable sugar comes from malt wort and the balance is made up by the three different syrups. The effects on the fermentability as can be seen are quite marked. The very low fermentability syrups such as the maltodextrin syrups described in Table 2 can be used in the production of low alcohol beers providing body and mouthfeel but very limited fermentable extract.