In starch science, the term “conversion” refers to depolymerization. Products that are partially depolymerized are called “converted starches.” There are three basic processes for making converted starches: using acid, using an oxidant in an alkaline system, and application of heat.
To make acid-modified, acid-converted, or thin-boiling starches, a slurry of starch granules is stirred with a mineral acid at a temperature below the starch’s gelatinization temperature. The acid catalyzes the hydrolysis of glycosidic linkages, but the hydrolysis is not random and (1-6) linkages are preferentially hydrolyzed over (1-4) linkages. The hydrolysis occurs more rapidly in the less dense, less crystalline regions of the granules. Once the desired degree of hydrolysis is reached, the suspension is neutralized, and the granules are recovered, washed, and dried. The hydrolysis pattern can vary, and it can either proceed from the outer granule surface inward or from the hilum region of the granule outward in a radial fashion, facilitated by channels within the granules.
The conversion of starch is stopped by monitoring the viscosity of a hot paste of the acid-modified starch. This is known as determining fluidity in the starch industry. Acid conversion can be done on both native and modified starches. Compared to the parent starch, acid-converted starches have reduced molecular weights, increased paste clarity, higher solubility in hot water, reduced hot paste viscosity, increased gel strength and opacity, and better film-forming capabilities. The specific characteristics of the converted starch depend on the parent starch and the conversion conditions.
Oxidation conversion breaks the chain of starch by introducing a carbonyl unit at C2, C3, or C6 of a glucopyranosyl unit. Sodium hypochlorite solution is commonly used for this process. The hypochlorite anion oxidizes the hydroxymethyl group (C6) to an aldehydo group, and the hydroxyl groups at C2 or C3 to keto groups, resulting in a dialdehyde. Oxidized starch can also be produced using hydrogen peroxide and copper(II) ions or ammonium persulfate for paper sizing and coating processes. The process is stopped by monitoring the hot paste viscosity or fluidity. Oxidized starch has different properties such as reduced molecular weight, increased solubility, and improved film-forming abilities.
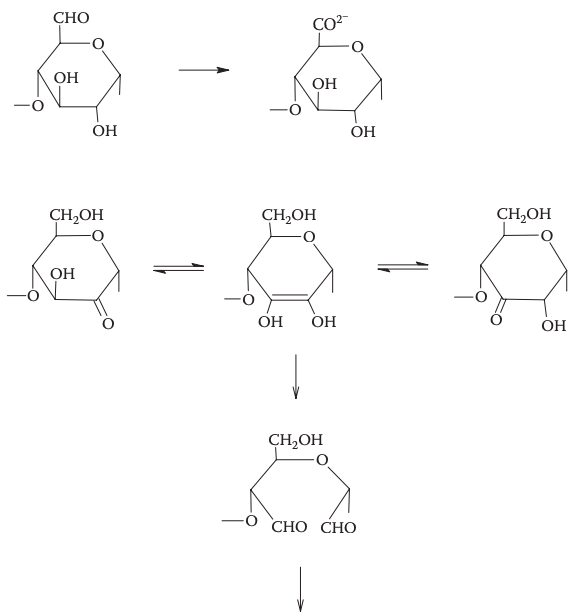
Researchers have suggested that the least organized part of starch granules is the central area around the hilum, based on evidence that reactions occur first in the center and progress outward. This is supported by the observation that digestion of ungelatinized granules with pores and channels starts in the central cavity region of the hilum and moves outward. Similar reactions, such as hypochlorite oxidation, are likely to occur in the same way.
Oxidative cleavage and acid-catalyzed hydrolysis are similar methods of converting starch, but there are two differences. Oxidized starches tend to make clearer and more stable pastes and gels than acid-modified starches because the larger carboxylate groups prevent retrogradation and repel like charges. Also, there is more variability in products that can be obtained through choice of parent starch, oxidizing agent, and conditions of oxidation. For example, using low levels of hypochlorite at an acidic pH can result in a product with a greater hot paste viscosity.
