Amylopectin is a large molecule (107 and 108 Da) with a high branching degree (Hanselmann et al., 1996). Several authors agree that the chain length distribution and the molar or weight ratio of amylopectin play an important role in starch functional properties. It has been suggested that, in some products elaborated with wheat starch, amylopectin fraction would be the main factor responsible for the functional characteristics of the final product (Kobayashi, Schwartz, and Lineback, 1986).
For example, amylopectin from three corn starches (amylose content of 36, 41, and 59%) with different proportions of short (DP 17–22) and long (DP 99–110) chains (Takeda, Takeda, and Hizukuri, 1993) present different gelatinization behavior. In amylopectin, the higher the proportion of short chains, the lower the gelatinization temperature is (Jane et al., 1999). Conversely, the amylopectin with a high level of long branching chains presents a high gelatinization temperature and a strong tendency to retrogradation (Jane et al., 1999; Chang and Lin, 2007).
As with amylose, amylopectin structure is usually evaluated by chromatographic methods. The first structural studies of the chain length distribution of amylopectin were carried out using gel filtration chromatography (Bio-Gel P-10, 100–200 mesh, Sepharose CL-2B) by debranching the chains with isoamylase or pullulanase (Biliaderis, Grant, and Vose, 1981; Biliaderis, 1982; Hizukuri, 1986; Paredes-Lopez, Bello-Perez, and Lopez, 1994). The debranched components of starch were determined by the total sugar and reducing endgroup content (Dubois et al., 1956; Ong et al., 1994). The average degree of polymerization of the eluted fraction can be determined by dividing the total carbohydrate concentration (quantified by phenol-sulfuric acid) by its reducing capacity to obtain the molar ratios of chain populations (Biliaderis, 1982; Paredes-Lopez, Bello-Perez, and Lopez, 1994).
Debranched amylopectin from different species (potato, cassava, and waxy rice) evaluated by HPLC-RI shows polymodal distribution, indicating that B-chains are separated in different groups (B1, B2, B3, and B4) (Hizukuri, 1986). The proposed distribution is consistent with the cluster model, which best explains the amylopectin structure: chains B1 and A make a single cluster; chains in fractions B2 and B3 extend into two and three clusters, respectively; and chains in fraction B4 stretch across more than four clusters.
Amylopectin molecular weights from normal maize, smooth pea (Aberle et al., 1994), amaranth starch (Bello-Perez et al., 1998c), and waxy corn starch (Millard et al., 1997) were evaluated by the HPSEC-MALLS system. However, Rg is difficult to evaluate in amylopectin by the HPSECMALLS system because heterogeneities in chromatograms may be related to branching density of amylopectin. To better evaluate amylopectin structure by chromatographic methods, some studies have been focused on sample preparation, mainly on the solubilization step. Amylopectin isolated from normal maize solubilized by microwave heating for 35 seconds and analyzed by the HPSEC-MALLS-RI system, showed a Mw 20 × 10-7 g/mol and Rg of 227 nm (Bello-Perez et al., 1998b). However, samples show different chromatography profiles and molecular weight if solubilized with (Mw 2.2 × 108 g/mol) or without DMSO (Mw 9.4 × 107 g/mol) (Bello-Perez et al., 1998a). Concerning the typical botanic structure, an HPSEC profile of debranched cassava starches shows trimodal distribution and structural differences in these populations (Charles et al., 2005).
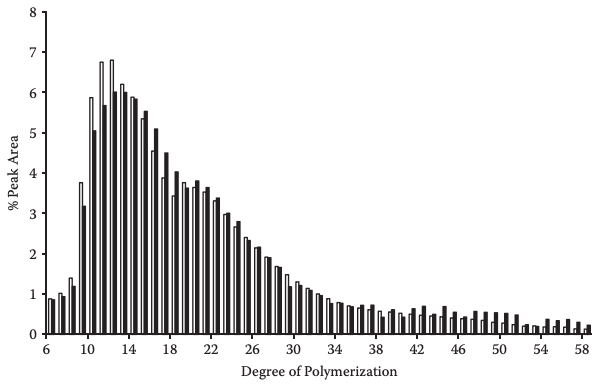
Ion exchange chromatography, specifically anion exchange chromatography (HPAEC), coupled with pulsed amperometric detector (PAD) has allowed us to learn the chain length distribution of the individual components of debranching amylopectin. Amylopectin from different botanical sources debranched with isoamylase shows chains between 10 and 13 degrees of polymerization (Koizumi, Fukuda, and Hizukuri, 1991; Suzuki et al., 1992). Comparisons of the debranching pattern of amylopectin from diverse botanical sources with isoamylase and pullulanase using the HPAEC-PAD equipment have been an effective technique for evaluating chain length distribution (Bello-Perez et al., 1996b). In four groups of chains in several cultivars of rice starch debranched with isoamylase, one was constituted of A-chains and the other three groups corresponded to the B-chains of the cluster model (Patindol and Wang, 2002). Figure 1 shows a modified HPAEC-PAD chromatographic profile obtained using a postcolumn amyloglucosidase reactor; this system was named HPAEC-ENZ-PAD (Wong and Jane, 1997). The chain length distribution of debranching amylopectin from soft wheat starches obtained by this technique was DP 25.6 and 26.9 and some cultivars had amylopectin with more long chains (DP ≥ 37) and longer average chain length (DP 26.2–26.9). The structural characteristics were related to the thermal and pasting pattern; the gelatinization temperature, paste peak viscosity, and shear thinning increased with increasing branch chain length of amylopectin (Franco et al., 2002).
Amylopectin isolated from bananas and analyzed by the HPAEC-ENZPAD system showed a higher proportion (24.0%) of longer branch chains (DP ≥ 37) than amylopectin from other botanical sources (normal and waxy maize and rice, barley, and waxy amaranth) (Jane et al., 1999). Using this technique it was also possible to correlate functional properties with morphological and structural characteristics on A- and B-type starch granules from cereal starches, indicating that large granules (A) have a higher proportion of longer chains than small granules (B) (Ao and Jane, 2007).
