Acid-thinned, thin-boiling, and fluidity starches are terms referring to starches that have been subjected to acid hydrolysis. The acid hydrolysis is carried out by using the electrophilic hydroxonium ion (H3O+) to attack the oxygen atom on the α-1,4 glycosidic bond and hydrolyze the glucosidic linkages. Then the electrons in one of the carbon-oxygen bonds move onto the oxygen atom and generate an unstable, high-energy carbocation intermediate. Subsequently, the carbocation intermediate reacts with water, leading to the regeneration of a hydroxyl group (Hoover, 2000). The cleavage of starch granule by acid hydrolysis starts from the granule surface to the interior and the amorphous regions of the starch granule are found to be more susceptible to acid hydrolysis than the crystalline regions (Jenkins and Donald, 1997).
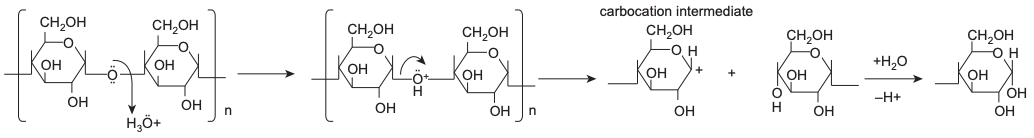
Some problems that might be encountered during acid hydrolysis are random attack at the branch point (which could lead to an increase in the linearity of starch), high glucose yield, and acid removal; hence, enzymatic hydrolysis is preferred over acid hydrolysis. Alternatively, the presence of long-chain alcohols helps to reduce the degree of polymerization of amylopectin more effectively and also convert the crystalline regions into more amorphous regions that are prone to acid hydrolysis (Singh et al., 2016). The residue after acid hydrolysis contains the starch nanocrystals, which have high crystallinity and nanoscale platelet morphology. The nanocrystal starches can be refined and preserved under evaluated conditions by completely removing the amorphous region of starch granules and not destroying the starch crystalline structure (Lin et al., 2011; Putaux et al., 2003).
